Development of a Current Collector with a Graphene Thin Film for a Proton Exchange Membrane Fuel Cell Module
- PMID: 32093390
- PMCID: PMC7070319
- DOI: 10.3390/molecules25040955
Development of a Current Collector with a Graphene Thin Film for a Proton Exchange Membrane Fuel Cell Module
Abstract
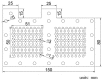
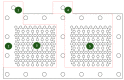
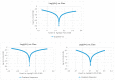
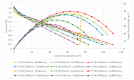
This paper constructs planar-type graphene thin film current collectors for proton exchange membrane fuel cells (PEMFCs). The present planar-type current collector adopts FR-4 as the substrate and coats a copper thin film using thermal evaporation for the electric-conduction layer. A graphene thin film is then coated onto the current collector to prevent corrosion due to electrochemical reactions. Three different coating techniques are conducted and compared: Spin coating, RF magnetron sputtering, and screen printing. The corrosion rates and surface resistances are tested and compared for the different coating techniques. Single cell PEMFCs with the developed current collectors are assembled and tested. A PEMFC module with two cells is also designed and constructed. The cell performances are measured to investigate the device feasibility.
Keywords: current collector; graphene thin film; module; proton exchange membrane fuel cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- O’Hayre R., Cha S.W., Colella W., Prinz F.B. Fuel Cell Fundamentals. 3rd ed. John Wiley & Sons; New York, NY, USA: 2016.
-
- Lee S.J., Chang-Chien A., Cha S.W., O’Hayre R., Park Y.I., Saito Y., Prinz F.B. Design and fabrication of a micro fuel cell array with flip-flop interconnection. J. Power Sources. 2002;110:410–418. doi: 10.1016/S0378-7753(02)00393-2. - DOI
-
- Cha H.Y., Choi H.G., Nam J.D., Lee Y., Cho S.M., Lee E.S., Lee J.K., Chung C.H. Fabrication of all- polymer micro-DMFCs using UV-sensitive photoresist. Electrochim. Acta. 2004;242:795–799. doi: 10.1016/j.electacta.2004.01.117. - DOI
-
- Lu G.Q., Wang C.Y., Yen T.J., Zhang X. Development and characterization of a silicon-based micro direct methanol fuel cell. Electrochim. Acta. 2004;49:821–828. doi: 10.1016/j.electacta.2003.09.036. - DOI
-
- Yun Y.H. Deposition of gold-titanium and gold-nickel coatings on electro polished 316L stainless steel bipolar plates for proton exchange membrane fuel cells. J. Hydrogen Energy. 2010;35:1713–1718. doi: 10.1016/j.ijhydene.2009.12.036. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

