Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells
- PMID: 32094077
- PMCID: PMC7102607
- DOI: 10.1016/j.resinv.2019.12.005
Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells
Abstract
Background: Coronavirus 229E (HCoV-229E), one of the causes of the common cold, exacerbates chronic obstructive pulmonary disease (COPD) and bronchial asthma. Long-acting muscarinic antagonists and β2-agonists and inhaled corticosteroids inhibit the exacerbation of COPD and bronchial asthma caused by infection with viruses, including HCoV-229E. However, the effects of these drugs on HCoV-229E replication and infection-induced inflammation in the human airway are unknown.
Methods: Primary human nasal (HNE) and tracheal (HTE) epithelial cell cultures were infected with HCoV-229E.
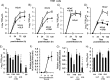
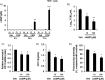
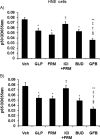
Results: Pretreatment of HNE and HTE cells with glycopyrronium or formoterol decreased viral RNA levels and/or titers, the expression of the HCoV-229E receptor CD13, the number and fluorescence intensity of acidic endosomes where HCoV-229E RNA enters the cytoplasm, and the infection-induced production of cytokines, including IL-6, IL-8, and IFN-β. Treatment of the cells with the CD13 inhibitor 2'2'-dipyridyl decreased viral titers. Pretreatment of the cells with a combination of three drugs (glycopyrronium, formoterol, and budesonide) exerted additive inhibitory effects on viral titers and cytokine production. Pretreatment of HNE cells with glycopyrronium or formoterol reduced the susceptibility to infection, and pretreatment with the three drugs inhibited activation of nuclear factor-kappa B p50 and p65 proteins. Pretreatment with formoterol increased cAMP levels and treatment with cAMP decreased viral titers, CD13 expression, and the fluorescence intensity of acidic endosomes.
Conclusions: These findings suggest that glycopyrronium, formoterol, and a combination of glycopyrronium, formoterol, and budesonide inhibit HCoV-229E replication partly by inhibiting receptor expression and/or endosomal function and that these drugs modulate infection-induced inflammation in the airway.
Keywords: Airway epithelial cells; CD13; HCoV-229E; Long-acting muscarinic antagonist; Long-acting β(2) agonist.
Copyright © 2020 The Japanese Respiratory Society. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest This work was supported by a research support grant from AstraZeneca KK (NCR-17-12892). Glycopyrronium, formoterol, and budesonide were obtained from AstraZeneca PLC.
Figures




References
-
- Shirato K., Kawase M., Watanabe O., Hirokawa C., Matsuyama S., Nishimura H. Differences in neutralizing antigenicity between laboratory and clinical isolates of HCoV-229E isolated in Japan in 2004- 2008 depend on the S1 region sequence of the spike protein. J Gen Virol. 2012;93:1908–1917. - PubMed
-
- Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

