Comparison of Treatment Outcomes between Analysis Populations in the RESTORE-IMI 1 Phase 3 Trial of Imipenem-Cilastatin-Relebactam versus Colistin plus Imipenem-Cilastatin in Patients with Imipenem-Nonsusceptible Bacterial Infections
- PMID: 32094127
- PMCID: PMC7179630
- DOI: 10.1128/AAC.02203-19
Comparison of Treatment Outcomes between Analysis Populations in the RESTORE-IMI 1 Phase 3 Trial of Imipenem-Cilastatin-Relebactam versus Colistin plus Imipenem-Cilastatin in Patients with Imipenem-Nonsusceptible Bacterial Infections
Abstract
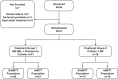
The RESTORE-IMI 1 phase 3 trial demonstrated the efficacy and safety of imipenem-cilastatin (IMI) combined with relebactam (REL) for treating imipenem-nonsusceptible infections. The objective of this analysis was to compare the outcomes among patients meeting eligibility requirements based on central laboratory susceptibility versus local laboratory susceptibility. Patients with serious infections caused by imipenem-nonsusceptible, colistin-susceptible, and imipenem-REL-susceptible pathogens were randomized 2:1 to IMI-REL plus placebo or colistin plus IMI for 5 to 21 days. The primary endpoint was a favorable overall response. Key endpoints included the clinical response and all-cause mortality. We compared outcomes between the primary microbiological modified intent-to-treat (mMITT) population, where eligibility was based on central laboratory susceptibility testing, and the supplemental mMITT (SmMITT) population, where eligibility was based on local, site-level testing. The SmMITT (n = 41) and MITT (n = 31) populations had similar baseline characteristics, including sex, age, illness severity, and renal function. In both analysis populations, favorable overall response rates in the IMI-REL treatment group were >70%. Favorable clinical response rates at day 28 were 71.4% for IMI-REL and 40.0% for colistin plus IMI in the mMITT population, whereas they were 75.0% for IMI-REL and 53.8% for colistin plus IMI in the SmMITT population. Day 28 all-cause mortality rates were 9.5% for IMI-REL and 30.0% for colistin plus IMI in the mMITT population, whereas they were 10.7% for IMI-REL and 23.1% for colistin plus IMI in the SmMITT population. The outcomes in the SmMITT population were generally consistent with those in the mMITT population, suggesting that outcomes may be applicable to the real-world use of IMI-REL for treating infections caused by imipenem-nonsusceptible Gram-negative pathogens. (This study has been registered at ClinicalTrials.gov under identifier NCT02452047.).
Keywords: carbapenem resistant; local microbiology data; supplemental analysis population; β-lactamase inhibitor.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re.... Accessed 16 January 2019.
-
- Cai B, Echols R, Magee G, Arjona Ferreira JC, Morgan G, Ariyasu M, Sawada T, Nagata TD. 2017. Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 4:ofx176. doi:10.1093/ofid/ofx176. - DOI - PMC - PubMed
-
- Martin A, Fahrbach K, Zhao Q, Lodise T. 2018. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis 5:ofy150. doi:10.1093/ofid/ofy150. - DOI - PMC - PubMed
-
- Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. 2017. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 17:279. doi:10.1186/s12879-017-2383-z. - DOI - PMC - PubMed
-
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi:10.1016/S1473-3099(13)70190-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

