The aging skin microenvironment dictates stem cell behavior
- PMID: 32094197
- PMCID: PMC7071859
- DOI: 10.1073/pnas.1901720117
The aging skin microenvironment dictates stem cell behavior
Abstract
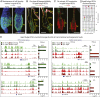
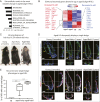
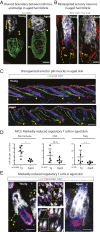
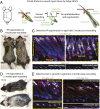
Aging manifests with architectural alteration and functional decline of multiple organs throughout an organism. In mammals, aged skin is accompanied by a marked reduction in hair cycling and appearance of bald patches, leading researchers to propose that hair follicle stem cells (HFSCs) are either lost, differentiate, or change to an epidermal fate during aging. Here, we employed single-cell RNA-sequencing to interrogate aging-related changes in the HFSCs. Surprisingly, although numbers declined, aging HFSCs were present, maintained their identity, and showed no overt signs of shifting to an epidermal fate. However, they did exhibit prevalent transcriptional changes particularly in extracellular matrix genes, and this was accompanied by profound structural perturbations in the aging SC niche. Moreover, marked age-related changes occurred in many nonepithelial cell types, including resident immune cells, sensory neurons, and arrector pili muscles. Each of these SC niche components has been shown to influence HF regeneration. When we performed skin injuries that are known to mobilize young HFSCs to exit their niche and regenerate HFs, we discovered that aged skin is defective at doing so. Interestingly, however, in transplantation assays in vivo, aged HFSCs regenerated HFs when supported with young dermis, while young HFSCs failed to regenerate HFs when combined with aged dermis. Together, our findings highlight the importance of SC:niche interactions and favor a model where youthfulness of the niche microenvironment plays a dominant role in dictating the properties of its SCs and tissue health and fitness.
Keywords: aging; hair follicle; lineage identity; skin; stem cells.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Till J. E., McCulloch E. A., A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 213–222 (1961). - PubMed
-
- Becker A. J., McCulloch E. A., Till J. E., Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197, 452–454 (1963). - PubMed
-
- Sharpless N. E., DePinho R. A., How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8, 703–713 (2007). - PubMed
-
- Rossi D. J., Jamieson C. H., Weissman I. L., Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

