Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits
- PMID: 32094203
- PMCID: PMC7117897
- DOI: 10.1523/JNEUROSCI.2402-19.2020
Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits
Abstract
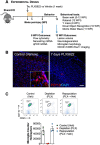
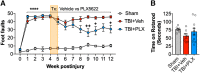
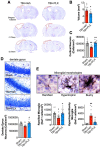
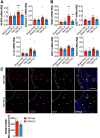
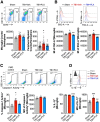
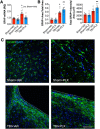
Chronic neuroinflammation with sustained microglial activation occurs following severe traumatic brain injury (TBI) and is believed to contribute to subsequent neurodegeneration and neurological deficits. Microglia, the primary innate immune cells in brain, are dependent on colony stimulating factor 1 receptor (CSF1R) signaling for their survival. In this preclinical study, we examined the effects of delayed depletion of chronically activated microglia on functional recovery and neurodegeneration up to 3 months postinjury. A CSF1R inhibitor, Plexxikon (PLX) 5622, was administered to adult male C57BL/6J mice at 1 month after controlled cortical impact to remove chronically activated microglia, and the inhibitor was withdrawn 1-week later to allow for microglial repopulation. Following TBI, the repopulated microglia displayed a ramified morphology similar to that of Sham uninjured mice, whereas microglia in vehicle-treated TBI mice showed the typical chronic posttraumatic hypertrophic morphology. PLX5622 treatment limited TBI-associated neuropathological changes at 3 months postinjury; these included a smaller cortical lesion, reduced hippocampal neuron cell death, and decreased NOX2- and NLRP3 inflammasome-associated neuroinflammation. Furthermore, delayed depletion of chronically activated microglia after TBI led to widespread changes in the cortical transcriptome and altered gene pathways involved in neuroinflammation, oxidative stress, and neuroplasticity. Using a variety of complementary neurobehavioral tests, PLX5622-treated TBI mice also had improved long-term motor and cognitive function recovery through 3 months postinjury. Together, these studies demonstrate that chronic phase removal of neurotoxic microglia after TBI using CSF1R inhibitors markedly reduce chronic neuroinflammation and associated neurodegeneration, as well as related motor and cognitive deficits.SIGNIFICANCE STATEMENT Traumatic brain injury (TBI) is a debilitating neurological disorder that can seriously impact the patient's quality of life. Microglial-mediated neuroinflammation is induced after severe TBI and contributes to neurological deficits and on-going neurodegenerative processes. Here, we investigated the effect of breaking the neurotoxic neuroinflammatory loop at 1-month after controlled cortical impact in mice by pharmacological removal of chronically activated microglia using a colony stimulating factor 1 receptor (CSF1R) inhibitor, Plexxikon 5622. Overall, we show that short-term elimination of microglia during the chronic phase of TBI followed by repopulation results in long-term improvements in neurological function, suppression of neuroinflammatory and oxidative stress pathways, and a reduction in persistent neurodegenerative processes. These studies are clinically relevant and support new concepts that the therapeutic window for TBI may be far longer than traditionally believed if chronic and evolving microglial-mediated neuroinflammation can be inhibited or regulated in a precise manner.
Keywords: CSF1R; functional recovery; microglia; neurodegeneration; neuroinflammation; traumatic brain injury.
Copyright © 2020 the authors.
Figures


Comment in
-
Does Delayed Microglial Ablation Alter Outcomes after Traumatic Brain Injury?J Neurosci. 2020 Oct 21;40(43):8211-8213. doi: 10.1523/JNEUROSCI.1336-20.2020. J Neurosci. 2020. PMID: 33087459 Free PMC article. No abstract available.
References
-
- Babior BM. (1999) NADPH oxidase: an update. Blood 93:1464–1476. - PubMed
-
- Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P, Perdoux J, Perrot L, Nash M, Desrayaud S, Wipfli P, Frieauff W, Shimshek DR (2018) Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol Commun 6:9. 10.1186/s40478-018-0510-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
