Generation and Evaluation of a Glaesserella (Haemophilus) parasuis Capsular Mutant
- PMID: 32094250
- PMCID: PMC7171231
- DOI: 10.1128/IAI.00879-19
Generation and Evaluation of a Glaesserella (Haemophilus) parasuis Capsular Mutant
Abstract
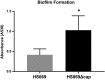
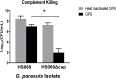
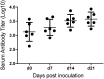
Glaesserella (Haemophilus) parasuis is a commensal bacterium of the upper respiratory tract in pigs and also the causative agent of Glässer's disease, which causes significant morbidity and mortality in pigs worldwide. Isolates are characterized into 15 serovars by their capsular polysaccharide, which has shown a correlation with isolate pathogenicity. To investigate the role the capsule plays in G. parasuis virulence and host interaction, a capsule mutant of the serovar 5 strain HS069 was generated (HS069Δcap) through allelic exchange following natural transformation. HS069Δcap was unable to cause signs of systemic disease during a pig challenge study and had increased sensitivity to complement killing and phagocytosis by alveolar macrophages. Compared with the parent strain, HS069Δcap produced more robust biofilm and adhered equivalently to 3D4/31 cells; however, it was unable to persistently colonize the nasal cavity of inoculated pigs, with all pigs clearing HS069Δcap by 5 days postchallenge. Our results indicate the importance of the capsular polysaccharide to G. parasuis virulence as well as nasal colonization in pigs.
Keywords: Glaesserella parasuis; Glässer’s disease; acapsular mutant; capsular polysaccharide.
Copyright © 2020 American Society for Microbiology.
Figures





References
-
- Aragon V, Segales J, Oliveira S. 2012. Glasser’s disease, p 760–769. In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW (ed), Diseases of swine, 10th ed John Wiley & Sons, Inc, West Sussex, United Kingdom.
-
- Howell KJ, Peters SE, Wang J, Hernandez-Garcia J, Weinert LA, Luan SL, Chaudhuri RR, Angen O, Aragon V, Williamson SM, Parkhill J, Langford PR, Rycroft AN, Wren BW, Maskell DJ, Tucker AW; BRaDP1T Consortium. 2015. Development of a multiplex PCR assay for rapid molecular serotyping of Haemophilus parasuis. J Clin Microbiol 53:3812–3821. doi:10.1128/JCM.01991-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- BB/G019177/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G020744/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 109385/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- BB/G018553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G019274/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

