Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis
- PMID: 32094304
- PMCID: PMC7046955
- DOI: 10.1136/rmdopen-2019-001117
Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis
Abstract
Background: Biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs are used in patients with psoriatic arthritis (PsA), but few studies directly compare their clinical efficacy. In such situations, network meta-analysis (NMA) can inform evidence-based decision-making.
Objective: To evaluate the comparative efficacy and safety of approved bDMARDs in patients with PsA.
Methods: Bayesian NMA was conducted to compare the clinical efficacy of bDMARDs at weeks 12‒16 in bDMARD-naïve patients with PsA in terms of American College of Rheumatology (ACR) criteria, Psoriatic Arthritis Response Criteria (PsARC) and Psoriasis Area and Severity Index (PASI). Safety end points were evaluated in the overall mixed population of bDMARD-naive and bDMARD-experienced patients.
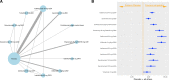
Results: For ACR, all treatments except abatacept were statistically superior to placebo. Infliximab was most effective, followed by golimumab and etanercept, which were statistically superior to most other treatments. Ixekizumab 80 mg every 2 weeks (Q2W) was statistically superior to abatacept subcutaneous, apremilast and both regimens of ustekinumab; similar findings were observed for ixekizumab 80 mg Q4W. For PsARC response, ixekizumab did not significantly differ from other therapies, except for golimumab, infliximab and etanercept, which were superior to most other agents including ixekizumab. For PASI response, infliximab was numerically most effective, but was not statistically superior to ixekizumab, which was the next best performing agent. Analysis of safety end points identified few differences between treatments.
Conclusion: Our NMA confirms the efficacy and acceptable safety profile of bDMARDs in patients with active PsA. There were generally few statistically significant differences between most treatments.
Keywords: DMARDs (biologic); DMARDs (synthetic); psoriatic arthritis.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AR-W has received honoraria for conferences and as a scientific expert from Abbvie, Amgen, Biogen, BMS, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi and UCB. RP and GB are full-time employees of Adelphi Values Ltd, and CW of their cooperation partner, Clarostat Consulting Ltd, who were commissioned by Eli Lilly and Company to conduct the analysis for this work. SL-L, GK, CS and SH are full-time employees of Eli Lilly and Company, receive a salary and own company stock. DN and SK were full-time employees of Eli Lilly and Company during the inception of the work.
Figures


References
-
- Mease PJ, Smolen JS, Behrens F, et al. . A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
