Meningeal lymphatic vessels regulate brain tumor drainage and immunity
- PMID: 32094452
- PMCID: PMC7054407
- DOI: 10.1038/s41422-020-0287-8
Meningeal lymphatic vessels regulate brain tumor drainage and immunity
Abstract
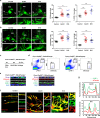
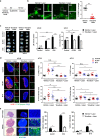
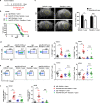
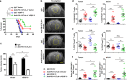
Recent studies have shown that meningeal lymphatic vessels (MLVs), which are located both dorsally and basally beneath the skull, provide a route for draining macromolecules and trafficking immune cells from the central nervous system (CNS) into cervical lymph nodes (CLNs), and thus represent a potential therapeutic target for treating neurodegenerative and neuroinflammatory diseases. However, the roles of MLVs in brain tumor drainage and immunity remain unexplored. Here we show that dorsal MLVs undergo extensive remodeling in mice with intracranial gliomas or metastatic melanomas. RNA-seq analysis of MLV endothelial cells revealed changes in the gene sets involved in lymphatic remodeling, fluid drainage, as well as inflammatory and immunological responses. Disruption of dorsal MLVs alone impaired intratumor fluid drainage and the dissemination of brain tumor cells to deep CLNs (dCLNs). Notably, the dendritic cell (DC) trafficking from intracranial tumor tissues to dCLNs decreased in mice with defective dorsal MLVs, and increased in mice with enhanced dorsal meningeal lymphangiogenesis. Strikingly, disruption of dorsal MLVs alone, without affecting basal MLVs or nasal LVs, significantly reduced the efficacy of combined anti-PD-1/CTLA-4 checkpoint therapy in striatal tumor models. Furthermore, mice bearing tumors overexpressing VEGF-C displayed a better response to anti-PD-1/CTLA-4 combination therapy, and this was abolished by CCL21/CCR7 blockade, suggesting that VEGF-C potentiates checkpoint therapy via the CCL21/CCR7 pathway. Together, the results of our study not only demonstrate the functional aspects of MLVs as classic lymphatic vasculature, but also highlight that they are essential in generating an efficient immune response against brain tumors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Meningeal lymphatics "drain" brain tumors.Cell Res. 2020 Mar;30(3):191-192. doi: 10.1038/s41422-020-0286-9. Cell Res. 2020. PMID: 32111971 Free PMC article. No abstract available.
References
-
- Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18:123–131. - PubMed
-
- Shirai Y. On the transplantation of the rat sarcoma in adult heterogeneous animals. Jpn. Med. World. 1921;1:14–15.
-
- Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

