Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials
- PMID: 32094461
- PMCID: PMC7449872
- DOI: 10.1038/s41375-020-0756-6
Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials
Abstract
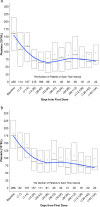
Selinexor is an oral, small molecule inhibitor of the nuclear export protein exportin 1 with demonstrated activity in hematologic and solid malignancies. Side effects associated with selinexor include nausea, vomiting, fatigue, diarrhea, decreased appetite, weight loss, thrombocytopenia, neutropenia, and hyponatremia. We reviewed 437 patients with multiple myeloma treated with selinexor and assessed the kinetics of adverse events and impact of supportive care measures. Selinexor reduced both platelets and neutrophils over the first cycle of treatment and reached a nadir between 28 and 42 days. Platelet transfusions and thrombopoietin receptor agonists were effective at treating thrombocytopenia, and granulocyte colony stimulating factors were effective at resolving neutropenia. The onset of gastrointestinal side effects (nausea, vomiting, and diarrhea) was most common during the first 1-2 weeks of treatment. Nausea could be mitigated with 5-HT3 antagonists and either neurokinin 1 receptor antagonists, olanzapine, or cannbainoids. Loperamide and bismuth subsalicylate ameliorated diarrhea. The primary constitutional side effects of fatigue and decreased appetite could be managed with methylphenidate, megestrol, cannabinoids or olanzapine, respectively. Hyponatremia was highly responsive to sodium replacement. Selinexor has well-established adverse effects that mainly occur within the first 8 weeks of treatment, are reversible, and respond to supportive care.
Conflict of interest statement
MG: honoraria from Amgen, Karyopharm, Takeda, Genesis, Janssen-Cilag. AC: grant support, advisory board fees, research funding, and/or consulting fees from Takeda, Celgene, Novartis, Amgen, Janssen, BMS, Sanofi, Oncopeptides, Pharmacyclics, Seattle Genetics, Array BioPharma. DV: consulting, research funding, and/or advisory role Celgene, Amgen, Karyopharm, Teva, Janssen, Millennium, Acetylon, GlaxoSmithKline, Calithera Biosciences, Constellation. AJ: research funding, honoraria, consulting, and/or advisory role Karyopharm, Amgen, Abbvie, Celgene, Janssen, Takeda, Sanofi, SkylineDx. DD: research funding Takeda, Karyopharm. CH: grant or personal fees Janssen, Celgene, Karyopharm, Oncopeptides, Adaptive Biotechnologies. DS: speakers’ bureau and/or funding: Celgene, Amgen, Janssen, Takeda, BMS. DW: consultancy fees and honoraria Amgen, Celgene, Janssen, Sanofi, Takeda. SL: advisor and/or shareholder Caelum Biosciences, Janssen, Bayer. CAH: fees/travel/advisory/and/or grant support Janssen, Celgene, Sanofi, Karyopharm, Glenmark. SJ: advisory or consulting fees Celgene, BMS, Janssen, Merck. RB: consulting, advisory role, and/or funding Celgene, Karyopharm, Signal Genetics, Takeda, Merck. AN: advisory board fees Amgen, GlaxoSmithKline, BMS, Celgene, Takeda, Janssen, Spectrum, Adaptive Biotechnologies. JR: consulting fees and speakers bureau Amgen, Celgene, Takeda, Janssen, Sanofi, Karyopharm, Oncopeptides, Adaptive Biotechnologies, and BMS. RA: honoraria, consulting, advisory role, and/or speaker’s bureau Celgene, Amgen, Takeda, Janssen, BMS, Karyopharm. AY: consulting fees or grant support Adaptive Biotechnologies, Amgen, Karyopharm, Takeda, Janssen, Dexcel Pharma, BMS, Celgene. PM: honoraria Janssen, Celgene, Takeda, Amgen, and AbbVie. SL: advisory board fees Celgene, Takeda, Janssen, Novartis, BMS, and GlaxoSmithKline. ST: consulting/speaker’s bureau/advisory board or grant support Celgene, Amgen, Janssen, Sanofi, Merck, Alnylam. KW: honoraria/consulting/advisory role/funding Amgen, BMS, Celgene, Janssen, Novartis, Onyx, Takeda, Sanofi. MM: grant support/fees Janssen, Sanofi, Jazz Celgene, BMS, Takeda Amgen, Roche. TU, KL, YC, LL, JS, SS, MK: employees/stockholders in Karyopharm. SS: Founder/President/CSO Karyopharm. MK: CEO Karyopharm. MD: honorarium/consulting/advisory role/funding Amgen, Novartis, Celgene, Takeda, Genesis, Janssen, BMS. The remaining authors report no relevant conflicts of interest.
Figures

References
-
- San Miguel JF, Hungria VTM, Yoon S-S, Beksac M, Dimopoulos MA, Elghandour A, et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016;3:e506–15. doi: 10.1016/S2352-3026(16)30147-8. - DOI - PubMed
-
- Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27. doi: 10.1016/S0140-6736(16)31594-X. - DOI - PMC - PubMed

