Elucidating the Electrochemical Mechanism of NG-Hydroxy-L-arginine
- PMID: 32095022
- PMCID: PMC7039656
- DOI: 10.1149/1945-7111/ab643a
Elucidating the Electrochemical Mechanism of NG-Hydroxy-L-arginine
Abstract
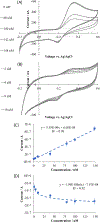
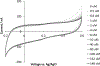
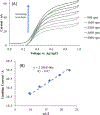
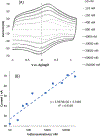
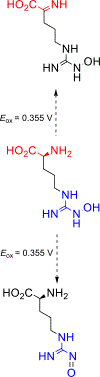
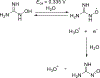
NG-Hydroxy-L-arginine (NOHA) is a stable intermediate product in the urea cycle that can be used to monitor the consumption of L-arginine by nitrous oxide synthase (NOS) to produce nitric oxide (NO) and L-citrulline. Research has implicated the urea cycle in many diseases and NO has cultivated interest as a potential biomarker for neural health. Electrochemical detection is an established, cost-effective method that can successfully detect low levels of analyte concentrations. As one of the few electrochemically active species in the urea cycle, NOHA shows promise as a biomarker for monitoring disruptions in this biochemical process. In this study, we show that NOHA has an oxidation peak at +355 mV vs Ag/AgCl at a glassy carbon electrode. In addition, cyclic voltammetry studies with structural analogs - alanine and N-hydroxyguanidine - allowed us to approximate the oxidation wave at +355 mV vs Ag/AgCl to be a one electron process. Diffusivity of NOHA was found using linear scan voltammetry with a rotating disk electrode and approximated at 5.50×10-5 cm2/s. Ample work is still needed to make a robust biosensor, but the results here characterize the electrochemical activity and represent principle steps in making a NOHA biosensor.
Figures



Similar articles
-
Electrochemical Detection of NG-Hydroxy-L-arginine.ECS Trans. 2018;85(13):1163-1169. doi: 10.1149/08513.1163ecst. ECS Trans. 2018. PMID: 30245763 Free PMC article.
-
Substrate specificity of NO synthases: detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine.Biochemistry. 1998 Jul 21;37(29):10453-60. doi: 10.1021/bi980742t. Biochemistry. 1998. PMID: 9671515
-
N-Aryl N'-hydroxyguanidines, a new class of NO-donors after selective oxidation by nitric oxide synthases: structure-activity relationship.J Med Chem. 2002 Feb 14;45(4):944-54. doi: 10.1021/jm011006h. J Med Chem. 2002. PMID: 11831907
-
Oxidation of N-hydroxyguanidines by cytochromes P450 and NO-synthases and formation of nitric oxide.Drug Metab Rev. 2002 Aug;34(3):593-606. doi: 10.1081/dmr-120005661. Drug Metab Rev. 2002. PMID: 12214669 Review.
-
Alternative nitric oxide-producing substrates for NO synthases.Free Radic Biol Med. 2004 Oct 15;37(8):1105-21. doi: 10.1016/j.freeradbiomed.2004.06.031. Free Radic Biol Med. 2004. PMID: 15451052 Review.
Cited by
-
The emergence of psychoanalytical electrochemistry: the translation of MDD biomarker discovery to diagnosis with electrochemical sensing.Transl Psychiatry. 2022 Sep 8;12(1):372. doi: 10.1038/s41398-022-02138-y. Transl Psychiatry. 2022. PMID: 36075922 Free PMC article. Review.
-
Application of nano-radiosensitizers in combination cancer therapy.Bioeng Transl Med. 2023 Feb 10;8(3):e10498. doi: 10.1002/btm2.10498. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206240 Free PMC article. Review.
-
Redox state and metabolic responses to severe heat stress in lenok Brachymystax lenok (Salmonidae).Front Mol Biosci. 2023 May 24;10:1156310. doi: 10.3389/fmolb.2023.1156310. eCollection 2023. Front Mol Biosci. 2023. PMID: 37293553 Free PMC article.
References
-
- Moncada S, Palmer RMJ, and Higgs EA, Biochem. Pharmacol, 38, 1709–1715 (1989). - PubMed
-
- Botchlett R, Lawler JM, and Wu G, Nutr. Enhanc. Sport. Perform, 645–652 (2019).
-
- Singh R, Pervin S, Karimi A, Cederbaum S, and Chaudhuri G, Cancer Res, 60, 3305–12 (2000). - PubMed
-
- Pervin S, Singh R, and Chaudhuri G, Nitric Oxide, 19, 103–106 (2008). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
