Hyaluronan Dermal Fillers: Efforts Towards a Wider Biophysical Characterization and the Correlation of the Biophysical Parameters to the Clinical Outcome
- PMID: 32095081
- PMCID: PMC6995295
- DOI: 10.2147/CCID.S220227
Hyaluronan Dermal Fillers: Efforts Towards a Wider Biophysical Characterization and the Correlation of the Biophysical Parameters to the Clinical Outcome
Abstract
Introduction: Hyaluronic Acid (HA) fillers are among the most used products in cosmetic medicine. Companies offer different formulations to allow full facial treatment and/or remodeling. Gels are being studied to establish the biophysical properties behind the specific clinical use and a correlation between the gel biophysical properties and their clinical performance. Clinicians' awareness is growing about the potential benefit deriving from such biophysical characterization.
Aim: The Aliaxin® line of HA dermal fillers is the object of this study. The study aimed to widen the biophysical characterization of these gels by investigating a variety of properties to better support their optimal use. Further, we aimed to provide some clinical findings to gain a deeper insight into the correlation between filler features and clinical outcome.
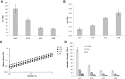
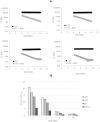
Methods: The four gels of the line were investigated, for the first time, for their cohesivity and stability to Reactive Oxygen Species (ROS). Additional secondary rheological parameters; evidence of relative water-uptake ability; and some clinical findings on product safety, palpability and duration of the aesthetic effect are provided.
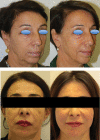
Results and conclusion: The gels proved highly cohesive and sensitive to ROS action with stability declining with the decrease in the overall gel elasticity. The G* and complex viscosity values at clinically relevant frequencies and gel water-uptake ability are consistent with the relative clinical indication related to gel projection and hydration capacity. Clinical outcomes showed the safety of the products and a perception of palpability well correlating with the cohesive/viscosity properties of the gels. A similar duration of the aesthetic effect (up to 1 year) was observed despite the diverse in vitro gel stability. The results broaden our knowledge of these gels and may contribute to optimize their clinical use towards the improvement of patient safety and satisfaction. Initial clinical observation indicated that gel biophysical properties allow for a reliable prediction of gel palpability, while in vitro data on gel stability cannot be related to the duration of the observed skin improvement. The latter finding further corroborates the idea of a skin restoration process activated by the gels besides the physical volumetric action.
Keywords: cohesivity; degradation; dermal filler; palpability.
© 2020 La Gatta et al.
Conflict of interest statement
The material for in vitro experiments has been provided by IBSA Farmaceutici Italia Srl. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Hyaluronan Hydrogels for Injection in Superficial Dermal Layers: An In Vitro Characterization to Compare Performance and Unravel the Scientific Basis of Their Indication.Int J Mol Sci. 2021 Jun 2;22(11):6005. doi: 10.3390/ijms22116005. Int J Mol Sci. 2021. PMID: 34199374 Free PMC article.
-
Hyaluronan-based hydrogels as dermal fillers: The biophysical properties that translate into a "volumetric" effect.PLoS One. 2019 Jun 11;14(6):e0218287. doi: 10.1371/journal.pone.0218287. eCollection 2019. PLoS One. 2019. PMID: 31185059 Free PMC article.
-
Biophysical and biological characterization of a new line of hyaluronan-based dermal fillers: A scientific rationale to specific clinical indications.Mater Sci Eng C Mater Biol Appl. 2016 Nov 1;68:565-572. doi: 10.1016/j.msec.2016.06.008. Epub 2016 Jun 7. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27524055
-
The science of hyaluronic acid dermal fillers.J Cosmet Laser Ther. 2008 Mar;10(1):35-42. doi: 10.1080/14764170701774901. J Cosmet Laser Ther. 2008. PMID: 18330796 Review.
-
Comparative physical properties of hyaluronic acid dermal fillers.Dermatol Surg. 2009 Feb;35 Suppl 1:302-12. doi: 10.1111/j.1524-4725.2008.01046.x. Dermatol Surg. 2009. PMID: 19207319 Review.
Cited by
-
Rheologic and Physicochemical Characteristics of Hyaluronic Acid Fillers: Overview and Relationship to Product Performance.Facial Plast Surg. 2022 Apr;38(2):116-123. doi: 10.1055/s-0041-1741560. Epub 2022 Feb 3. Facial Plast Surg. 2022. PMID: 35114708 Free PMC article. Review.
-
Elucidations on the Performance and Reversibility of Treatment with Hyaluronic Acid Based Dermal Fillers: In vivo and in vitro Approaches.Clin Cosmet Investig Dermatol. 2022 Dec 8;15:2629-2640. doi: 10.2147/CCID.S383354. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 36523543 Free PMC article.
-
Hyaluronan Hydrogels for Injection in Superficial Dermal Layers: An In Vitro Characterization to Compare Performance and Unravel the Scientific Basis of Their Indication.Int J Mol Sci. 2021 Jun 2;22(11):6005. doi: 10.3390/ijms22116005. Int J Mol Sci. 2021. PMID: 34199374 Free PMC article.
-
Spontaneous and induced degradation of dermal fillers: A review.J Cutan Aesthet Surg. 2024 Oct-Dec;17(4):273-281. doi: 10.4103/JCAS.JCAS_137_23. Epub 2023 Nov 30. J Cutan Aesthet Surg. 2024. PMID: 39649762 Free PMC article. Review.
-
Comprehensive Evaluation of Injectability Attributes in OxiFree™ Dermal Fillers: MaiLi® Product Variants and Clinical Case Reports.Gels. 2024 Apr 19;10(4):276. doi: 10.3390/gels10040276. Gels. 2024. PMID: 38667695 Free PMC article.
References
LinkOut - more resources
Full Text Sources

