Sequential Cohort Analysis After Liver Transplantation Shows de Novo Extended Release Tacrolimus Is Safe, Efficacious, and Minimizes Renal Dysfunction
- PMID: 32095514
- PMCID: PMC7004634
- DOI: 10.1097/TXD.0000000000000970
Sequential Cohort Analysis After Liver Transplantation Shows de Novo Extended Release Tacrolimus Is Safe, Efficacious, and Minimizes Renal Dysfunction
Abstract
The use of once-daily extended-release tacrolimus (ERT) is associated with improved long-term graft and patient survival when compared with twice-daily tacrolimus (BDT), but the underlying reasons for differential survival are unclear. The aim of the study was to compare clinical outcomes known to impact on posttransplant survival for de novo BDT and ERT in liver transplantation (LT) recipients.
Methods: We conducted a single-center, prospective sequential cohort analysis of adult patients undergoing LT during a change in protocol from de novo BDT to ERT, with a 6-month post-LT follow-up.
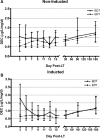
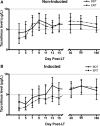
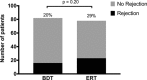
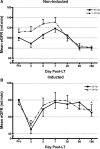
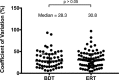
Results: A total of 160 transplanted patients were evaluated; 82 were in the BDT group and 78 were in the ERT group. The cohorts were matched for standard variables and a similar proportion in each group received induction interleukin-2 receptor antibody (36% and 31%). There were no significant differences in the measured outcomes of patient and graft survival, biopsy-proven acute rejection episodes, post LT diabetes, and toxicity. A significantly lower number of patients developed chronic kidney disease Stage3-4 in the ERT cohort compared with BDT cohort. In patients with pre-LT renal dysfunction who received antibody induction, estimated glomerular filtration rate decreased significantly in the BDT but not the ERT group.
Conclusions: We show that once-daily ERT is as safe and efficacious as BDT in de novo LT but optimally conserves renal function post-LT.
Copyright © 2020 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures
Similar articles
-
Favorable longterm outcomes of liver transplant recipients treated de novo with once-daily tacrolimus: Results of a single-center cohort.Liver Transpl. 2016 Oct;22(10):1391-400. doi: 10.1002/lt.24514. Liver Transpl. 2016. PMID: 27434676
-
Early tacrolimus exposure does not impact long-term outcomes after liver transplantation.World J Hepatol. 2021 Mar 27;13(3):362-374. doi: 10.4254/wjh.v13.i3.362. World J Hepatol. 2021. PMID: 33815678 Free PMC article.
-
Efficacy and Safety of Delayed Prolonged-Release Tacrolimus Initiation in De Novo Hepatitis C Virus-Negative Orthotopic Liver Transplant Recipients: A Single-Center, Single-Arm, Prospective Study.Ann Transplant. 2019 Jan 18;24:36-44. doi: 10.12659/AOT.912444. Ann Transplant. 2019. PMID: 30655498 Free PMC article.
-
Once-daily prolonged-release tacrolimus versus twice-daily tacrolimus in liver transplantation.J Am Pharm Assoc (2003). 2019 Nov-Dec;59(6):816-823.e2. doi: 10.1016/j.japh.2019.08.002. Epub 2019 Sep 11. J Am Pharm Assoc (2003). 2019. PMID: 31521585
-
Once-Daily versus Twice-Daily Tacrolimus in Kidney Transplantation: A Systematic Review and Meta-analysis of Observational Studies.Drugs. 2019 Dec;79(18):1947-1962. doi: 10.1007/s40265-019-01217-7. Drugs. 2019. PMID: 31713065 Free PMC article.
Cited by
-
A Systematic Review of the Literature on Chronic Kidney Disease Following Liver Transplantation.Ann Transplant. 2022 May 24;27:e935170. doi: 10.12659/AOT.935170. Ann Transplant. 2022. PMID: 35607264 Free PMC article.
References
-
- Group EFMLS Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994; 344: 423–428 - PubMed
-
- Group TUSMFLS A Comparison of Tacrolimus (FK 506) and Cyclosporine for Immunosuppression in Liver Transplantation. N Engl Journal of Medicine. 1994; 331: 1110–1115 - PubMed
-
- Advagraf European Public Assessment Reports (EPAR) 2007 Scientific Discussion. 2007Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_D.... Accessed December 2018
-
- Adam R, Karam V, Delvart V, et al. European Liver Intestine Transplant Association (ELITA) Improved survival in liver transplant recipients receiving prolonged-release tacrolimus in the european liver transplant registry. Am J Transplant. 2015; 15: 1267–1282 - PubMed
LinkOut - more resources
Full Text Sources

