Sustainable Low-Concentration Arsenite [As(III)] Removal in Single and Multicomponent Systems Using Hybrid Iron Oxide-Biochar Nanocomposite Adsorbents-A Mechanistic Study
- PMID: 32095682
- PMCID: PMC7033674
- DOI: 10.1021/acsomega.9b02842
Sustainable Low-Concentration Arsenite [As(III)] Removal in Single and Multicomponent Systems Using Hybrid Iron Oxide-Biochar Nanocomposite Adsorbents-A Mechanistic Study
Abstract
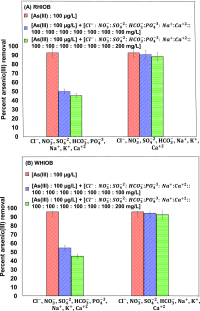
Rice and wheat husks were converted to biochars by slow pyrolysis (1 h) at 600 °C. Iron oxide rice husk hybrid biochar (RHIOB) and wheat husk hybrid biochar (WHIOB) were synthesized by copyrolysis of FeCl3-impregnated rice or wheat husks at 600 °C. These hybrid sorbents were characterized using X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy (SEM), SEM-energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, transmission electron microscopy, physical parameter measurement system, and Brunauer-Emmett-Teller (BET) surface area techniques. Fe3O4 was the predominant iron oxide present with some Fe2O3. RHIOB and WHIOB rapidly chemisorbed As(III) from water (∼24% removal in first half an hour reaching up to ∼100% removal in 24 h) at surface Fe-OH functions forming monodentate ≡Fe-OAs(OH)2 and bidentate (≡Fe-O)2AsOH complexes. Optimum removal occurred in the pH 7.5-8.5 range for both RHIOB and WHIOB, but excellent removal occurred from pH 3 to 10. Batch kinetic studies at various initial adsorbate-adsorbent concentrations, temperatures, and contact times gave excellent pseudo-second-order model fits. Equilibrium data were fitted to different sorption isotherm models. Fits to isotherm models (based on R 2 and χ2) on RHIOB and WHIOB followed the order: Redlich-Peterson > Toth > Sips = Koble-Corrigan > Langmuir > Freundlich = Radke-Prausnitz > Temkin and Sips = Koble-Corrigan > Toth > Redlich-Peterson > Langmuir > Temkin > Freundlich = Radke-Prausnitz, respectively. Maximum adsorption capacities, Q RHIOB 0 = 96 μg/g and Q WHIOB 0 = 111 μg/g, were obtained. No As(III) oxidation to As(V) was detected. Arsenic adsorption was endothermic. Particle diffusion was a rate-determining step at low (≤50 μg/L) concentrations, but film diffusion controls the rate at ≥100-200 μg/L. Binding interactions with RHIOB and WHIOB were established, and the mechanism was carefully discussed. RHIOB and WHIOB can successfully be used for As(III) removal in single and multicomponent systems with no significant decrease in adsorption capacity in the presence of interfering ions mainly Cl-, HCO3 -, NO3 -, SO4 2-, PO4 3-, K+, Na+, Ca2+. Simultaneous As(III) desorption and regeneration of RHIOB and WHIOB was successfully achieved. A very nominal decrease in As(III) removal capacity in four consecutive cycles demonstrates the reusability of RHIOB and WHIOB. Furthermore, these sustainable composites had good sorption efficiencies and may be removed magnetically to avoid slow filtration.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures













References
-
- Ahmaruzzaman M.; Gupta V. K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. 10.1021/ie201477c. - DOI
-
- Sahu S. J.; Nath B.; Roy S.; Mandal B.; Chatterjee D. A laboratory batch study on arsenic sorption and desorption on guava orchard soils of Baruipur, West Bengal, India. J. Geochem. Explor. 2011, 108, 157–162. 10.1016/j.gexplo.2011.01.003. - DOI
-
- Chen W.-Q.; Shi Y.-L.; Wu S.-L.; Zhu Y.-G. Anthropogenic arsenic cycles: A research framework and features. J. Clean. Prod. 2016, 139, 328–336. 10.1016/j.jclepro.2016.08.050. - DOI
-
- Hafeznezami S.; Zimmer-Faust A. G.; Jun D.; Rugh M. B.; Haro H. L.; Park A.; Suh J.; Najm T.; Reynolds M. D.; Davis J. A.; Parhizkar T.; Jay J. A. Remediation of groundwater contaminated with arsenic through enhanced natural attenuation: Batch and column studies. Water Res. 2017, 122, 545–556. 10.1016/j.watres.2017.06.029. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
