Need for Digital Biomarkers in Musculoskeletal Trials
- PMID: 32095748
- PMCID: PMC7015349
- DOI: 10.1159/000479753
Need for Digital Biomarkers in Musculoskeletal Trials
Abstract
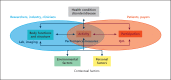
Pain and loss of function - both problems cause patients to visit a musculoskeletal specialist. Therefore, both lead symptoms should serve as a benchmark for new therapeutic approaches. New technologies generating digital biomarkers have the potential to significantly change musculoskeletal trials. However, more work is needed to agree upon data and variable standards, to improve user friendliness, and to ensure data integrity along the whole processing way. Therefore, rigorous and systematic testing of new technological approaches is required to establish new outcome variables suitable for musculoskeletal trials. Consortia of researchers working on similar technologies and outcome variables should collaborate from the beginning to enable comparing and pooling data. Early interaction with health authorities and regulatory bodies are necessary to pave the way for a widespread use of a new technology.
Keywords: Digital biomarker; Musculoskeletal trial.
Copyright © 2017 by S. Karger AG, Basel.
Figures
References
-
- Nightingale EJ, Pourkazemi F, Hiller CE. Systematic review of timed stair tests. J Rehabil Res Dev. 2014;51:335–350. - PubMed
-
- Bisca GW, Morita AA, Hernandes NA, Probst VS, Pitta F. Simple lower limb functional tests in patients with chronic obstructive pulmonary disease: a systematic review. Arch Phys Med Rehabil. 2015;96:2221–2230. - PubMed
-
- Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

