Digital Medicine: A Primer on Measurement
- PMID: 32095767
- PMCID: PMC7015383
- DOI: 10.1159/000500413
Digital Medicine: A Primer on Measurement
Abstract
Technology is changing how we practice medicine. Sensors and wearables are getting smaller and cheaper, and algorithms are becoming powerful enough to predict medical outcomes. Yet despite rapid advances, healthcare lags behind other industries in truly putting these technologies to use. A major barrier to entry is the cross-disciplinary approach required to create such tools, requiring knowledge from many people across many fields. We aim to drive the field forward by unpacking that barrier, providing a brief introduction to core concepts and terms that define digital medicine. Specifically, we contrast "clinical research" versus routine "clinical care," outlining the security, ethical, regulatory, and legal issues developers must consider as digital medicine products go to market. We classify types of digital measurements and how to use and validate these measures in different settings. To make this resource engaging and accessible, we have included illustrations and figures throughout that we hope readers will borrow from liberally. This primer is the first in a series that will accelerate the safe and effective advancement of the field of digital medicine.
Keywords: Clinical care; Clinical research; Cybersecurity; Data governance; Data rights; Decentralized clinical trials; Digital biomarkers; Digital health; Digital medicine; Digital technology tools; Ethics; FDA; Mobile technology; Privacy; Regulatory science; Research ethics; Sensors.
Copyright © 2019 by S. Karger AG, Basel.
Conflict of interest statement
Andrea Coravos is a founder and owns a significant interest in Elektra Labs Inc. Jennifer C. Goldsack is employed by monARC Bionetworks Inc. Daniel Karlin is a paid employee and/or owns a significant interest in HealthMode, CEAi, NightWare, Hu-manity.co, and 4YouandMe. Camille Nebeker is employed by UC San Diego School of Medicine and directs the ReCODE center. Eric Perakslis is affiliated with Harvard Medical School, Duke University, Elektra Labs Inc., and NuMedii Inc. Noah Zimmerman holds equity in Picnic Health. Kelley Erb is a paid employee of Pfizer Inc.
Figures
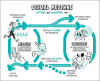
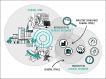









References
-
- Canavan N. Cartoons offer a peek into cancer immunotherapy — and scientists' minds. STAT. 2018 Oct 17; Available from: https://www.statnews.com/2018/10/17/cancer-immunotherapy-scientists-cart...
-
- Delp C, Jones J. Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions. Acad Emerg Med. 1996 Mar;3((3)):264–70. - PubMed
-
- The Digital Medicine Society The Digital Medicine (DiMe) Society. 2019 Available from: www.dimesociety.org.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

