Base and Covariate Population Pharmacokinetic Analyses of Dupilumab Using Phase 3 Data
- PMID: 32096596
- PMCID: PMC7496533
- DOI: 10.1002/cpdd.780
Base and Covariate Population Pharmacokinetic Analyses of Dupilumab Using Phase 3 Data
Abstract
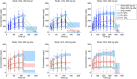
Population pharmacokinetic base and covariate models were developed to study functional dupilumab for regulatory submissions, using data from healthy volunteers and patients with moderate-to-severe atopic dermatitis (AD) receiving intravenous or subcutaneous doses. Sixteen studies were pooled (N = 2115; 202 healthy volunteers, 1913 AD patients). The best model was a 2-compartment model with linear and Michaelis-Menten elimination and 3 transit compartments describing absorption. A stepwise approach to model building, with some parameters estimated using mostly rich data and subsequently fixed, was used to avoid adverse effects of sparse data and a steep target-mediated phase on pharmacokinetic parameters, which require rich sampling for proper estimation. Parameterization of models in terms of rates was a useful alternative to the parameterization in terms of clearances, allowing for a reduced number of covariates while providing accurate predictions. While antidrug antibodies, albumin, race, body mass index, and Eczema Area and Severity Index score were statistically significant covariates, only body weight had a notable effect on central volume, explaining interindividual variability. The model adequately described dupilumab pharmacokinetics in phase 3 trials.
Keywords: atopic dermatitis; dupilumab; monoclonal antibodies; phase 3; population pharmacokinetics; regulatory submissions.
© 2020 Regeneron Pharmaceuticals, Inc. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
P.K., J.D.D., M.A., B.S., N.M.H.G., M.A.K., and A.T.D. are employees and shareholders of Regeneron Pharmaceuticals, Inc. R.R. was an employee and shareholder of Regeneron Pharmaceuticals, Inc. at the time of study conduct. G.P. and M.L. were employees of Sanofi at the time of study conduct and held stock and/or stock options in the company.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

