Na+/K+-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives
- PMID: 32096998
- PMCID: PMC7243443
- DOI: 10.1021/acs.jnatprod.9b01060
Na+/K+-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives
Abstract
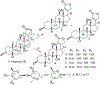
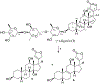
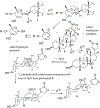
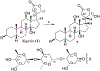
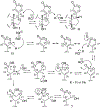
(+)-Digoxin (1) is a well-known cardiac glycoside long used to treat congestive heart failure and found more recently to show anticancer activity. Several known cardenolides (2-5) and two new analogues, (+)-8(9)-β-anhydrodigoxigenin (6) and (+)-17-epi-20,22-dihydro-21α-hydroxydigoxin (7), were synthesized from 1 and evaluated for their cytotoxicity toward a small panel of human cancer cell lines. A preliminary structure-activity relationship investigation conducted indicated that the C-12 and C-14 hydroxy groups and the C-17 unsaturated lactone unit are important for 1 to mediate its cytotoxicity toward human cancer cells, but the C-3 glycosyl residue seems to be less critical for such an effect. Molecular docking profiles showed that the cytotoxic 1 and the noncytotoxic derivative 7 bind differentially to Na+/K+-ATPase. The HO-12β, HO-14β, and HO-3'aα hydroxy groups of (+)-digoxin (1) may form hydrogen bonds with the side-chains of Asp121 and Asn122, Thr797, and Arg880 of Na+/K+-ATPase, respectively, but the altered lactone unit of 7 results in a rotation of its steroid core, which depotentiates the binding between this compound and Na+/K+-ATPase. Thus, 1 was found to inhibit Na+/K+-ATPase, but 7 did not. In addition, the cytotoxic 1 did not affect glucose uptake in human cancer cells, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na+/K+-ATPase but not by interacting with glucose transporters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Diederich M; Muller F; Cerella C Biochem. Pharmacol 2017, 125, 1–11. - PubMed
-
- Botelho AFM; Pierezan F; Soto-Blanco B; Melo MM Toxicon 2019, 158, 63–68. - PubMed
-
- Smith S J. Chem. Soc 1930, 508–510.
-
- Go K; Kartha G; Chen JP Acta Cryst. 1980, B36, 1811–1819.
-
- Ambrosy AP; Butler J; Ahmed A; Vaduganathan M; van Veldhuisen DJ; Colucci WS; Gheorghiade M J. Am. Coll. Cardiol 2014, 63, 1823–1832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

