Phase Ib/II Clinical Trial of Pembrolizumab With Bevacizumab for Metastatic Renal Cell Carcinoma: BTCRC-GU14-003
- PMID: 32097091
- PMCID: PMC7145584
- DOI: 10.1200/JCO.19.02394
Phase Ib/II Clinical Trial of Pembrolizumab With Bevacizumab for Metastatic Renal Cell Carcinoma: BTCRC-GU14-003
Abstract
Purpose: We hypothesized that bevacizumab will potentiate activity of pembrolizumab. We conducted a phase Ib/II, single-arm, multisite clinical trial of the combination in metastatic renal cell carcinoma (RCC).
Patients and methods: Patients with metastatic clear cell RCC who experienced progression after at least one systemic therapy (phase Ib) or were treatment naïve (phase II) were enrolled. In phase Ib, pembrolizumab (200 mg) and bevacizumab (10 or 15 mg/kg) were given intravenously every 3 weeks. The primary end point for phase II was overall response rate (ORR). With an 80% statistical power and a type I error probability of 0.1, 48 patients were to be accrued to detect an ORR of 42%.
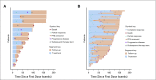
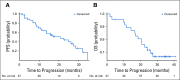
Results: Thirteen patients (ages 33-68 years; median, 55 years) were enrolled in the phase Ib study. No dose-limiting toxicities were reported. Pembrolizumab 200 mg and bevacizumab 15 mg/kg were chosen for phase II. Forty-eight patients (ages 42-84 years; median age, 61 years; 33 males) were accrued for the phase II study. The primary end point was met, with the ORR reaching 60.9% (95% CI, 45.4% to 74.9%), consisting of 1 complete response (CR), 2 CRs in target lesions, 25 partial responses, 18 responses of stable disease, 2 unevaluable responses. Median progression-free survival was 20.7 months (95% CI, 11.3 to 27.4 months). Median overall survival was not reached at the median follow-up of 28.3 months. The most common treatment-related grade 3 toxicities were hypertension and proteinuria. There were two grade 4 toxicities: duodenal ulcer and hyponatremia. Presence of tumor-infiltrating T cells, but not programmed death-ligand 1 expression, in tumor tissue correlated with response.
Conclusion: The combination of 200 mg of pembrolizumab and a 15 mg/kg dose of bevacizumab given every 3 weeks is safe and active in metastatic RCC.
Trial registration: ClinicalTrials.gov NCT02348008.
Figures
References
-
- Wu NZ, Klitzman B, Dodge R, et al. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–4268. - PubMed
-
- Marigo I, Dolcetti L, Serafini P, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. - PubMed
-
- Lewis JS, Landers RJ, Underwood JC, et al. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous