Virological suppression and clinical management in response to viremia in South African HIV treatment program: A multicenter cohort study
- PMID: 32097428
- PMCID: PMC7041795
- DOI: 10.1371/journal.pmed.1003037
Virological suppression and clinical management in response to viremia in South African HIV treatment program: A multicenter cohort study
Abstract
Background: Uptake of antiretroviral treatment (ART) is expanding rapidly in low- and middle-income countries (LMIC). Monitoring of virological suppression is recommended at 6 months of treatment and annually thereafter. In case of confirmed virological failure, a switch to second-line ART is indicated. There is a paucity of data on virological suppression and clinical management of patients experiencing viremia in clinical practice in LMIC. We report a large-scale multicenter assessment of virological suppression over time and management of viremia under programmatic conditions.
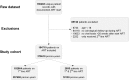
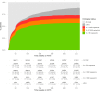
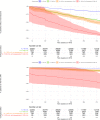
Methods and findings: Linked medical record and laboratory source data from adult patients on first-line ART at 52 South African centers between 1 January 2007 and 1 May 2018 were studied. Virological suppression, switch to second-line ART, death, and loss to follow-up were analyzed. Multistate models and Cox proportional hazard models were used to assess suppression over time and predictors of treatment outcomes. A total of 104,719 patients were included. Patients were predominantly female (67.6%). Median age was 35.7 years (interquartile range [IQR]: 29.9-43.0). In on-treatment analysis, suppression below 1,000 copies/mL was 89.0% at month 12 and 90.4% at month 72. Suppression below 50 copies/mL was 73.1% at month 12 and 77.5% at month 72. Intention-to-treat suppression was 75.0% and 64.3% below 1,000 and 50 copies/mL at month 72, respectively. Viremia occurred in 19.8% (20,766/104,719) of patients during a median follow-up of 152 (IQR: 61-265) weeks. Being male and below 35 years of age and having a CD4 count below 200 cells/μL prior to start of ART were risk factors for viremia. After detection of viremia, confirmatory testing took 29 weeks (IQR: 16-54). Viral resuppression to below 1,000 copies/mL without switch of ART occurred frequently (45.6%; 6,030/13,210) but was associated with renewed viral rebound and switch. Of patients with confirmed failure who remained in care, only 41.5% (1,872/4,510) were switched. The median time to switch was 68 weeks (IQR: 35-127), resulting in 12,325 person-years spent with a viral load above 1,000 copies/mL. Limitations of this study include potential missing data, which is in part addressed by the use of cross-matched laboratory source data, and the possibility of unmeasured confounding.
Conclusions: In this study, 90% virological suppression below the threshold of 1,000 copies/mL was observed in on-treatment analysis. However, this target was not met at the 50-copies/mL threshold or in intention-to-treat analysis. Clinical management in response to viremia was profoundly delayed, prolonging the duration of viremia and potential for transmission. Diagnostic tools to establish the cause of viremia are urgently needed to accelerate clinical decision-making.
Conflict of interest statement
AMJW, LEH, and MN report funding from The Netherlands Organization for Health Research and Development (ZonMW/WOTRO) during the study. AMJW further reports grants and consultancy fees from Janssen, Merck, ViiV Healthcare, and Gilead; grants and nonfinancial support from CLJI; and nonfinancial support and other from Virology Education, outside the submitted work, all paid to the University Medical Center Utrecht.
Figures

References
-
- UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. [cited 2020 Feb 6]. Available from: https://www.unaids.org/en/resources/documents/2017/90-90-90
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: Progress towards the 90-90-90 targets. 2017. [cited 2020 Feb 6]. Available from: http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_u...
-
- Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–82. 10.1016/S0140-6736(17)32340-1 - DOI - PubMed
-
- Gisslén M, Svedhem V, Lindborg L, Flamholc L, Norrgren H, Wendahl S, et al. Sweden, the first country to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization (WHO) 90-90-90 continuum of HIV care targets. HIV Med. 2017;18(4):305–7. 10.1111/hiv.12431 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

