Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain
- PMID: 32097464
- PMCID: PMC7201924
- DOI: 10.1084/jem.20191660
Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain
Abstract
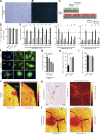
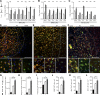
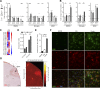
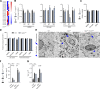
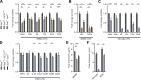
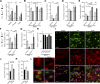
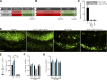
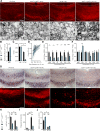
Failure of remyelination underlies the progressive nature of demyelinating diseases such as multiple sclerosis. Macrophages and microglia are crucially involved in the formation and repair of demyelinated lesions. Here we show that myelin uptake temporarily skewed these phagocytes toward a disease-resolving phenotype, while sustained intracellular accumulation of myelin induced a lesion-promoting phenotype. This phenotypic shift was controlled by stearoyl-CoA desaturase-1 (SCD1), an enzyme responsible for the desaturation of saturated fatty acids. Monounsaturated fatty acids generated by SCD1 reduced the surface abundance of the cholesterol efflux transporter ABCA1, which in turn promoted lipid accumulation and induced an inflammatory phagocyte phenotype. Pharmacological inhibition or phagocyte-specific deficiency of Scd1 accelerated remyelination ex vivo and in vivo. These findings identify SCD1 as a novel therapeutic target to promote remyelination.
© 2020 Bogie et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Alberti S., Schuster G., Parini P., Feltkamp D., Diczfalusy U., Rudling M., Angelin B., Björkhem I., Pettersson S., and Gustafsson J.A.. 2001. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J. Clin. Invest. 107:565–573. 10.1172/JCI9794 - DOI - PMC - PubMed
-
- Amar M.J., D’Souza W., Turner S., Demosky S., Sviridov D., Stonik J., Luchoomun J., Voogt J., Hellerstein M., Sviridov D., and Remaley A.T.. 2010. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther. 334:634–641. 10.1124/jpet.110.167890 - DOI - PMC - PubMed
-
- Anderson E.K., Hill A.A., and Hasty A.H.. 2012. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 32:1687–1695. 10.1161/ATVBAHA.112.250142 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

