CAP-MAP: cap analysis protocol with minimal analyte processing, a rapid and sensitive approach to analysing mRNA cap structures
- PMID: 32097574
- PMCID: PMC7058934
- DOI: 10.1098/rsob.190306
CAP-MAP: cap analysis protocol with minimal analyte processing, a rapid and sensitive approach to analysing mRNA cap structures
Abstract
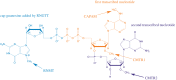
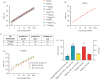
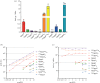
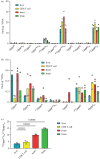
Eukaryotic messenger RNA (mRNA) is modified by the addition of an inverted guanosine cap to the 5' triphosphate. The cap guanosine and initial transcribed nucleotides are further methylated by a series of cap methyltransferases to generate the mature cap structures which protect RNA from degradation and recruit proteins involved in RNA processing and translation. Research demonstrating that the cap methyltransferases are regulated has generated interest in determining the methylation status of the mRNA cap structures present in cells. Here, we present CAP-MAP: cap analysis protocol with minimal analyte processing, a rapid and sensitive method for detecting cap structures present in mRNA isolated from tissues or cultured cells.
Keywords: 7-methylguanosine; RNA cap; RNA methylation; RNA processing; mass spectrometry; ribose O-2 methylation.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
