Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5
- PMID: 32097629
- PMCID: PMC7255387
- DOI: 10.1016/j.neuron.2020.01.030
Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5
Abstract
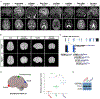
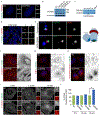
Genes mutated in human neuronal migration disorders encode tubulin proteins and a variety of tubulin-binding and -regulating proteins, but it is very poorly understood how these proteins function together to coordinate migration. Additionally, the way in which regional differences in neocortical migration are controlled is completely unknown. Here we describe a new syndrome with remarkably region-specific effects on neuronal migration in the posterior cortex, reflecting de novo variants in CEP85L. We show that CEP85L is required cell autonomously in vivo and in vitro for migration, that it localizes to the maternal centriole, and that it forms a complex with many other proteins required for migration, including CDK5, LIS1, NDE1, KIF2A, and DYNC1H1. Loss of CEP85L disrupts CDK5 localization and activation, leading to centrosome disorganization and disrupted microtubule cytoskeleton organization. Together, our findings suggest that CEP85L highlights a complex that controls CDK5 activity to promote neuronal migration.
Keywords: CDK5; CEP85L; Centrosome; De novo; Lissencephaly; Pachygyria; genetics; neurodevelopment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests C.A.W. serves on advisory boards for the Allen Brain Institute, Third Rock Ventures, and Maze Therapeutics and on editorial boards for Annals of Neurology, Trends in Neurosciences, and neuroDEVELOPMENTS. M.S. received research funding from Roche, Novartis, Pfizer, LAM Therapeutics, and Quadrant Biosciences; has served on the scientific advisory boards of Sage Therapeutics, Roche, Takeda, Celgene, and the PTEN Research Foundation; and serves on the Board of the Tuberous Sclerosis Alliance. All of these activities are outside of the submitted manuscript. J.S.C. is a consultant for Invitae. D.V. serves as a consultant to SK Life Science and Otsuka Pharmaceuticals, is on the speaker’s bureaus for UCB and Greenwich Pharmaceuticals, and conducts industry-supported clinical drug trials for SK Life Science, Biogen, and UCB Pharmaceuticals. K.M. is an employee of GeneDX, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

