Divalent cation influx and calcium homeostasis in germinal vesicle mouse oocytes
- PMID: 32097818
- PMCID: PMC7153984
- DOI: 10.1016/j.ceca.2020.102181
Divalent cation influx and calcium homeostasis in germinal vesicle mouse oocytes
Abstract
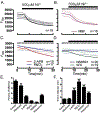
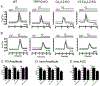
Prior to maturation, mouse oocytes are arrested at the germinal vesicle (GV) stage during which they experience constitutive calcium (Ca2+) influx and spontaneous Ca2+ oscillations. The oscillations cease during maturation but Ca2+ influx continues, as the oocytes' internal stores attain maximal content at the culmination of maturation, the metaphase II stage. The identity of the channel(s) that underlie this Ca2+ influx has not been completely determined. GV and matured oocytes are known to express three Ca2+ channels, CaV3.2, TRPV3 and TRPM7, but females null for each of these channels are fertile and their oocytes display minor modifications in Ca2+ homeostasis, suggesting a complex regulation of Ca2+ influx. To define the contribution of these channels at the GV stage, we used different divalent cations, pharmacological inhibitors and genetic models. We found that the three channels are active at this stage. CaV3.2 and TRPM7 channels contributed the majority of Ca2+ influx, as inhibitors and oocytes from homologous knockout (KO) lines showed severely reduced Ca2+ entry. Sr2+ influx was promoted by CaV3.2 channels, as Sr2+ oscillations were negligible in CaV3.2-KO oocytes but robust in control and Trpv3-KO GV oocytes. Mn2+ entry relied on expression of CaV3.2 and TRPM7 channels, but Ni2+ entry depended on the latter. CaV3.2 and TRPV3 channels combined to fill the Ca2+ stores, although CaV3.2 was the most impactful. Studies with pharmacological inhibitors effectively blocked the influx of divalent cations, but displayed off-target effects, and occasionally agonist-like properties. In conclusion, GV oocytes express channels mediating Ca2+ and other divalent cation influx that are pivotal for fertilization and early development. These channels may serve as targets for intervention to improve the success of assisted reproductive technologies.
Keywords: Calcium; Cav3.2; Influx; Meiosis; Signaling; TRPM7; TRPV3.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures






Similar articles
-
Deletion of TRPV3 and CaV3.2 T-type channels in mice undermines fertility and Ca2+ homeostasis in oocytes and eggs.J Cell Sci. 2021 Jul 1;134(13):jcs257956. doi: 10.1242/jcs.257956. Epub 2021 Jul 12. J Cell Sci. 2021. PMID: 34313315 Free PMC article.
-
TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization.Proc Natl Acad Sci U S A. 2018 Oct 30;115(44):E10370-E10378. doi: 10.1073/pnas.1810422115. Epub 2018 Oct 15. Proc Natl Acad Sci U S A. 2018. PMID: 30322909 Free PMC article.
-
Constitutive IP3R1-mediated Ca2+ release reduces Ca2+ store content and stimulates mitochondrial metabolism in mouse GV oocytes.J Cell Sci. 2019 Feb 12;132(3):jcs225441. doi: 10.1242/jcs.225441. J Cell Sci. 2019. PMID: 30659110 Free PMC article.
-
Cation channels in oocytes and early states of development: a novel type of nonselective cation channel activated by adrenaline in a clonal mesoderm-like cell line (MES-1).EXS. 1993;66:297-303. doi: 10.1007/978-3-0348-7327-7_24. EXS. 1993. PMID: 7505660 Review.
-
The mammalian melastatin-related transient receptor potential cation channels: an overview.Pflugers Arch. 2005 Oct;451(1):204-11. doi: 10.1007/s00424-005-1428-0. Epub 2005 May 14. Pflugers Arch. 2005. PMID: 15895246 Review.
Cited by
-
Deletion of TRPV3 and CaV3.2 T-type channels in mice undermines fertility and Ca2+ homeostasis in oocytes and eggs.J Cell Sci. 2021 Jul 1;134(13):jcs257956. doi: 10.1242/jcs.257956. Epub 2021 Jul 12. J Cell Sci. 2021. PMID: 34313315 Free PMC article.
-
Zn2+ is Essential for Ca2+ Oscillations in Mouse Eggs.bioRxiv [Preprint]. 2023 Nov 8:2023.04.13.536745. doi: 10.1101/2023.04.13.536745. bioRxiv. 2023. Update in: Elife. 2023 Dec 15;12:RP88082. doi: 10.7554/eLife.88082. PMID: 37131581 Free PMC article. Updated. Preprint.
-
Effects of meiotic stage-specific oocyte vitrification on mouse oocyte quality and developmental competence.Front Endocrinol (Lausanne). 2023 Jun 26;14:1200051. doi: 10.3389/fendo.2023.1200051. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37455899 Free PMC article.
-
The metallic compound promotes primordial follicle activation and ameliorates fertility deficits in aged mice.Theranostics. 2023 May 21;13(10):3131-3148. doi: 10.7150/thno.82553. eCollection 2023. Theranostics. 2023. PMID: 37351158 Free PMC article.
-
TRPM7 channel activity in Jurkat T lymphocytes during magnesium depletion and loading: implications for divalent metal entry and cytotoxicity.Pflugers Arch. 2020 Nov;472(11):1589-1606. doi: 10.1007/s00424-020-02457-3. Epub 2020 Sep 22. Pflugers Arch. 2020. PMID: 32964285 Free PMC article.
References
-
- Berridge MJ, Lipp P, and Bootman MD, The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol, 2000. 1(1): p. 11–21. - PubMed
-
- Cuthbertson KS, Whittingham DG, and Cobbold PH, Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature, 1981. 294(5843): p. 754–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

