Advances into understanding metabolites as signaling molecules in cancer progression
- PMID: 32097832
- PMCID: PMC7298879
- DOI: 10.1016/j.ceb.2020.01.013
Advances into understanding metabolites as signaling molecules in cancer progression
Abstract
Recent years have seen a great expansion in our knowledge of the roles that metabolites play in cellular signaling. Structural data have provided crucial insights into mechanisms through which amino acids are sensed. New nutrient-coupled protein and RNA modifications have been identified and characterized. A growing list of functions has been ascribed to metabolic regulation of modifications such as acetylation, methylation, and glycosylation. A current challenge lies in developing an integrated understanding of the roles that metabolic signaling mechanisms play in physiology and disease, which will inform the design of strategies to target such mechanisms. In this brief article, we review recent advances in metabolic signaling through post-translational modification during cancer progression, to provide a framework for understanding signaling roles of metabolites in the context of cancer biology and illuminate areas for future investigation.
Keywords: Cancer; Metabolic signaling; Metabolism.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures
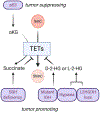
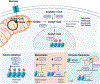
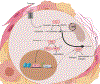
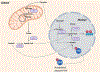
References
-
- Campbell SL, Wellen KE: Metabolic Signaling to the Nucleus in Cancer. Molecular Cell 2018, 71:398–408. - PubMed
-
- Lawrence RE, Zoncu R: The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol 2019, 21:133–142. - PubMed
-
- Chandel NS: Evolution of Mitochondria as Signaling Organelles. Cell Metabolism 2015, 22:204–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

