Dissecting the signaling pathways involved in the function of sperm flagellum
- PMID: 32097833
- PMCID: PMC7296462
- DOI: 10.1016/j.ceb.2020.01.015
Dissecting the signaling pathways involved in the function of sperm flagellum
Abstract
The mammalian flagellum is a specific type of motile cilium required for sperm motility and male fertility. Effective flagellar movement is dependent on axonemal function, which in turn relies on proper ion homeostasis within the flagellar compartment. This ion homeostasis is maintained by the concerted function of ion channels and transporters that initiate signal transduction pathways resulting in motility changes. Advances in electrophysiology and super-resolution microscopy have helped to identify and characterize new regulatory modalities of the mammalian flagellum. Here, we discuss what is currently known about the regulation of flagellar ion channels and transporters that maintain sodium, potassium, calcium, and proton homeostasis. Identification of new regulatory elements and their specific roles in sperm motility is imperative for improving diagnostics of male infertility.
Keywords: Capacitation; CatSper; EFCAB9; Fertility; Flagellum; Hv1; Motility; Progesterone; Slo1; Slo3; Sperm ion channels; pH.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: PVL has a financial interest in YourChoice Therapeutics, Inc.
Figures
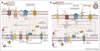

References
-
- Austin CR: Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 1951, 4:581–596. - PubMed
-
- Chang MC: Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168:697–698. - PubMed
-
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS: Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121:1139–1150. - PubMed
-
- Witte TS, Schäfer-Somi S: Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Anim Reprod Sci 2007, 102:181–193. - PubMed
-
- Dan Jean C: Studies on the acrosome. III. Effect of calcium deficiency. Biol Bull 1954, 107:335–349.

