Interactome and F-Actin Interaction Analysis of Dictyostelium discoideum Coronin A
- PMID: 32098122
- PMCID: PMC7073074
- DOI: 10.3390/ijms21041469
Interactome and F-Actin Interaction Analysis of Dictyostelium discoideum Coronin A
Abstract
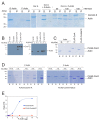
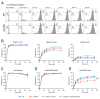
Coronin proteins are evolutionary conserved WD repeat containing proteins that have been proposed to carry out different functions. In Dictyostelium, the short coronin isoform, coronin A, has been implicated in cytoskeletal reorganization, chemotaxis, phagocytosis and the initiation of multicellular development. Generally thought of as modulators of F-actin, coronin A and its mammalian homologs have also been shown to mediate cellular processes in an F-actin-independent manner. Therefore, it remains unclear whether or not coronin A carries out its functions through its capacity to interact with F-actin. Moreover, the interacting partners of coronin A are not known. Here, we analyzed the interactome of coronin A as well as its interaction with F-actin within cells and in vitro. Interactome analysis showed the association with a diverse set of interaction partners, including fimbrin, talin and myosin subunits, with only a transient interaction with the minor actin10 isoform, but not the major form of actin, actin8, which was consistent with the absence of a coronin A-actin interaction as analyzed by co-sedimentation from cells and lysates. In vitro, however, purified coronin A co-precipitated with rabbit muscle F-actin in a coiled-coil-dependent manner. Our results suggest that an in vitro interaction of coronin A and rabbit muscle actin may not reflect the cellular interaction state of coronin A with actin, and that coronin A interacts with diverse proteins in a time-dependent manner.
Keywords: Actin; Dictyostelium; coronin A; interactome analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- de Hostos E.L. A brief history of the coronin family. Subcell. Biochem. 2008;48:31–40. - PubMed
-
- Goode B.L., Wong J.J., Butty A.C., Peter M., McCormack A.L., Yates J.R., Drubin D.G., Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

