Analysis of Single Circulating Tumor Cells in Renal Cell Carcinoma Reveals Phenotypic Heterogeneity and Genomic Alterations Related to Progression
- PMID: 32098246
- PMCID: PMC7073151
- DOI: 10.3390/ijms21041475
Analysis of Single Circulating Tumor Cells in Renal Cell Carcinoma Reveals Phenotypic Heterogeneity and Genomic Alterations Related to Progression
Abstract
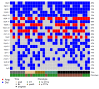
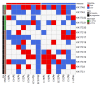
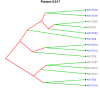
Circulating tumor cells (CTCs) are promising biomarkers for prognosis, therapeutic response prediction, and treatment monitoring in cancer patients. Despite its epithelial origin, renal cell carcinoma (RCC) shows low expression of epithelial markers hindering CTC-enrichment approaches exploiting epithelial cell surface proteins. In 21 blood samples serially collected from 10 patients with metastatic RCC entering the TARIBO trial, we overcame this limitation using the marker-independent Parsortix™ approach for CTC-enrichment coupled with positive and negative selection with the DEPArray™ with single cell recovery and analysis for copy number alterations (CNA) by next generation sequencing NGS. Two CTC subpopulations were identified: epithelial CTC (eCTC) and non-conventional CTC (ncCTC) lacking epithelial and leukocyte markers. With a threshold ≥1CTC/10 mL of blood, the positivity rates were 28% for eCTC, 62% for ncCTCs, and 71% considering both CTC types. In two patients with detectable eCTCs at baseline, progression free survival was less than 5 months. In an index case, hierarchical structure by translational oncology (TRONCO) identified three clones among 14 CTCs collected at progression and at baseline, each containing cells with a 9p21.3loss, a well-known metastasis driving subclonal alteration. CTCs detection in RCC can be increased by marker-independent approaches, and CTC molecular characterization can allow detection of subclonal events possibly related to tumor progression.
Keywords: cancer evolution; circulating tumor cells; copy number alterations; heterogeneity; renal cell cancer; single-cell analysis.
Conflict of interest statement
C.R. received two travel grants from Menarini Silicon Biosystems for attending the DEPArray User Meeting in Philadelphia, PA Sept 2018 and the 4th ACTC Meeting at Corfù, Greece October 2019. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Flanigan R.C., Yonover P.M. The role of radical nephrectomy in metastatic renal cell carcinoma. Semin. Urol. Oncol. 2001;19:98–102. - PubMed
-
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S., Negrier S., Szczylik C., Pili R., Bjarnason G.A., et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. - DOI - PMC - PubMed
-
- Sternberg C.N., Davis I.D., Mardiak J., Szczylik C., Lee E., Wagstaff J., Barrios C.H., Salman P., Gladkov O.A., Kavina A., et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. - DOI - PubMed
-
- Flanigan R.C., Salmon S.E., Blumenstein B.A., Bearman S.I., Roy V., McGrath P.C., Caton J.R., Jr., Munshi N., Crawford E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

