Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks
- PMID: 32098281
- PMCID: PMC7070556
- DOI: 10.3390/molecules25040978
Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks
Abstract
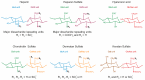
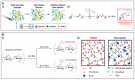
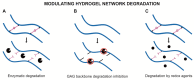
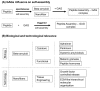
Glycosaminoglycans (GAG) are long, linear polysaccharides that display a wide range of relevant biological roles. Particularly, in the extracellular matrix (ECM) GAG specifically interact with other biological molecules, such as growth factors, protecting them from proteolysis or inhibiting factors. Additionally, ECM GAG are partially responsible for the mechanical stability of tissues due to their capacity to retain high amounts of water, enabling hydration of the ECM and rendering it resistant to compressive forces. In this review, the use of GAG for developing hydrogel networks with improved biological activity and/or mechanical properties is discussed. Greater focus is given to strategies involving the production of hydrogels that are composed of GAG alone or in combination with other materials. Additionally, approaches used to introduce GAG-inspired features in biomaterials of different sources will also be presented.
Keywords: GAG; GAG-mimetics; biomaterials; hybrid systems; hydrogels; polysaccharides; proteins; self-assembly peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- POCI-01-0145-FEDER-028744/FEDER - Fundo Europeu de Desenvolvimento Regional
- PTDC/BTMMAT/28744/2017/FCT - Fundação para a Ciência e a Tecnologia
- SFRH/BD/129855/2017/FCT - Fundação para a Ciência e a Tecnologia
- IF/00296/2015/FCT - Fundação para a Ciência e a Tecnologia
- POCI-01-0145-FEDER-032431/FEDER - Fundo Europeu de Desenvolvimento Regional
LinkOut - more resources
Full Text Sources
Other Literature Sources

