Vitamin D and Endothelial Function
- PMID: 32098418
- PMCID: PMC7071424
- DOI: 10.3390/nu12020575
Vitamin D and Endothelial Function
Abstract
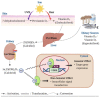
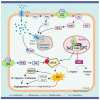
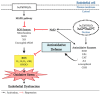
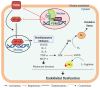
Vitamin D is known to elicit a vasoprotective effect, while vitamin D deficiency is a risk factor for endothelial dysfunction (ED). ED is characterized by reduced bioavailability of a potent endothelium-dependent vasodilator, nitric oxide (NO), and is an early event in the development of atherosclerosis. In endothelial cells, vitamin D regulates NO synthesis by mediating the activity of the endothelial NO synthase (eNOS). Under pathogenic conditions, the oxidative stress caused by excessive production of reactive oxygen species (ROS) facilitates NO degradation and suppresses NO synthesis, consequently reducing NO bioavailability. Vitamin D, however, counteracts the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase which produces ROS, and improves antioxidant capacity by enhancing the activity of antioxidative enzymes such as superoxide dismutase. In addition to ROS, proinflammatory mediators such as TNF-α and IL-6 are risk factors for ED, restraining NO and eNOS bioactivity and upregulating the expression of various atherosclerotic factors through the NF-κB pathway. These proinflammatory activities are inhibited by vitamin D by suppressing NF-κB signaling and production of proinflammatory cytokines. In this review, we discuss the diverse activities of vitamin D in regulating NO bioavailability and endothelial function.
Keywords: NO; NOX; ROS; calcitriol; eNOS; endothelial dysfunction; inflammation; nitric oxide; oxidative stress; vitamin D deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

