Determination of Eight Coccidiostats in Eggs by Liquid-Liquid Extraction-Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry
- PMID: 32098439
- PMCID: PMC7071118
- DOI: 10.3390/molecules25040987
Determination of Eight Coccidiostats in Eggs by Liquid-Liquid Extraction-Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry
Abstract
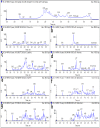
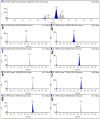
A method for the simultaneous determination of robenidine, halofuginone, lasalocid, monensin, nigericin, salinomycin, narasin, and maduramicin residues in eggs by liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed. The sample preparation method used a combination of liquid-liquid extraction (LLE) and solid-phase extraction (SPE) technology to extract and purify these target compounds from eggs. The target compounds were separated by gradient elution using high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC). Tandem mass spectrometry was used to quantitatively and qualitatively analyze the target compounds via electrospray ionization (ESI+) and multiple reaction monitoring mode. The HPLC-MS/MS and UPLC-MS/MS methods were validated according to the requirements defined by the European Union and the Food and Drug Administration. The limits of detection and limits of quantification of the eight coccidiostats in eggs were 0.23-0.52 µg/kg and 0.82-1.73 µg/kg for HPLC-MS/MS, and 0.16-0.42 µg/kg and 0.81-1.25 µg/kg for UPLC-MS/MS, respectively. The eggs were spiked with four concentrations of the eight coccidiostats, and the HPLC-MS/MS and UPLC-MS/MS average recoveries were all higher than 71.69% and 72.26%, respectively. Compared with the HPLC-MS/MS method, utilizing UPLC-MS/MS had the advantages of low reagent consumption, a short detection time, and high recovery and precision. Finally, the HPLC-MS/MS and UPLC-MS/MS methods were successfully applied to detect eight coccidiostats in 40 eggs.
Keywords: HPLC–MS/MS; LLE; SPE; UPLC–MS/MS; coccidiostats; eggs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Fard M.H.B., Rajab A. Evaluation of anti-coccidial vaccines and coccidiostate drugs on growth performance in experimental coccidiosis of broiler chickens; Proceedings of the EPC 2006-12th European Poultry Conference; Verona, Italy. 10–14 September 2006; World’s Poultry Science Association (WPSA)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

