Iridium(I)-Catalyzed α-C(sp3)-H Alkylation of Saturated Azacycles
- PMID: 32098471
- PMCID: PMC7219544
- DOI: 10.1021/jacs.9b12320
Iridium(I)-Catalyzed α-C(sp3)-H Alkylation of Saturated Azacycles
Abstract
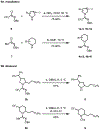
Saturated azacycles are commonly encountered in bioactive compounds and approved therapeutic agents. The development of methods for functionalization of the α-methylene C-H bonds of these highly privileged building blocks is of great importance, especially in drug discovery. While much effort has been dedicated toward this goal by using a directed C-H activation approach, the development of directing groups that are both general as well as practical remains a significant challenge. Herein, the design and development of novel amidoxime directing groups is described for Ir(I)-catalyzed α-C(sp3)-H alkylation of saturated azacycles using readily available olefins as coupling partners. This protocol extends the scope of saturated azacycles to piperidines, azepane, and tetrahydroisoquinoline that are incompatible with our previously reported directing group. A variety of olefin coupling partners, including previously unreactive disubstituted terminal olefins and internal olefins, are compatible with this transformation. The selectivity for a branched α-C(sp3)-alkylation product is also observed for the first time when acrylate is used as the reaction partner. The development of practical, one-step installation and removal protocols further adds to the utility of amidoxime directing groups.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



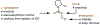
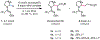
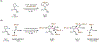

References
-
- Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845. - PubMed
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257. - PubMed
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752. - PubMed
- Murray CW; Rees DC Opportunity Knocks: Organic Chemistry for Fragment-Based Drug Discovery (FBDD). Angew. Chem. Int. Ed 2016, 55, 488. - PubMed
- Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem 2018, 10, 383. - PubMed
-
- Jovel I; Prateeptongkum S; Jackstell R; Vogl N; Weckbecker C; Beller M α-Functionalization of Non-activated Aliphatic Amines: Ruthenium-catalyzed Alkynylations and Alkylations. Chem. Commun 2010, 46, 1956. - PubMed
- Dai C; Meschini F; Narayanam JMR; Stephenson CRJ Friedel–Crafts Amidoalkylation via Thermolysis and Oxidative Photocatalysis. J. Org. Chem 2012, 77, 4425. - PMC - PubMed
- Girard SA; Knauber T; Li C-J The Cross-Dehydrogenative Coupling of C–H Bonds: A Versatile Strategy for C–C Bond Formations. Angew. Chem. Int. Ed 2014, 53, 74. - PubMed
- Seidel D The Azomethine Ylide Route to Amine C–H Functionalization: Redox-Versions of Classic Reactions and a Pathway to New Transformations. Acc. Chem. Res 2015, 48, 317. - PMC - PubMed
- Shang M; Chan JZ; Cao M; Chang Y; Wang Q; Cook B; Torker S; Wasa M C–H Functionalization of Amines via Alkene-Derived Nucleophiles through Cooperative Action of Chiral and Achiral Lewis Acid Catalysts: Applications in Enantioselective Synthesis. J. Am. Chem. Soc 2018, 140, 10593. - PMC - PubMed
-
- Meyers AI; Edwards PD; Rieker WF; Bailey TR Alpha-amino Carbanions via Formamidines. Alkylation of Pyrrolidines, Piperidines, and Related Heterocycles. J. Am. Chem. Soc 1984, 106, 3270.
- Beak P; Kerrick ST; Wu S; Chu J Complex Induced Proximity Effects: Enantioselective Syntheses Based on Asymmetric Deprotonations of N-Boc-pyrrolidines. J. Am. Chem. Soc 1994, 116, 3231.
- Campos KR; Klapars A; Waldman JH; Dormer PG; Chen C-Y Enantioselective, Palladium-Catalyzed α-Arylation of N-Bocpyrrolidine. J. Am. Chem. Soc 2006, 128, 3538. - PubMed
- Beng TK; Gawley RE Highly Enantioselective Catalytic Dynamic Resolution of N-Boc-2-lithiopiperidine: Synthesis of (R)-(+)-N-Boc-Pipecolic Acid, (S)-(−)-Coniine, (S)-(+)-Pelletierine, (+)-β-Conhydrine, and (S)-(−)Ropivacaine and Formal Synthesis of (−)-Lasubine II and (+)-Cermizine C. J. Am. Chem. Soc 2010, 132, 12216. - PMC - PubMed
- Hodgson DM; Kloesges J Lithiation–Electrophilic Substitution of NThiopivaloylazetidine. Angew. Chem. Int. Ed 2010, 49, 2900. - PubMed
- Seel S; Thaler T; Takatsu K; Zhang C; Zipse H; Straub BF; Mayer P; Knochel P Highly Diastereoselective Arylations of Substituted Piperidines. J. Am. Chem. Soc 2011, 133, 4774. - PubMed
- Cordier CJ; Lundgren RJ; Fu GC Enantioconvergent Cross-Couplings of Racemic Alkylmetal Reagents with Unactivated Secondary Alkyl Electrophiles: Catalytic Asymmetric Negishi α-Alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc 2013, 135, 10946. - PMC - PubMed
- Liniger M; Estermann K; Altmann K-H Total Synthesis of Hygrolines and Pseudohygrolines. J. Org. Chem 2013, 78, 11066. - PubMed
- Firth JD; O’Brien P; Ferris L Synthesis of Enantiopure Piperazines via Asymmetric Lithiation–Trapping of N-Boc Piperazines: Unexpected Role of the Electrophile and Distal N-Substituent. J. Am. Chem. Soc 2016, 138, 651. - PubMed
- Lin W; Zhang K-F; Baudoin O Regiodivergent enantioselective C–H functionalization of Boc-1,3-oxazinanes for the synthesis of β2-and β3amino acids. Nat. Catal 2019, 2, 882. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

