SPD_1495 Contributes to Capsular Polysaccharide Synthesis and Virulence in Streptococcus pneumoniae
- PMID: 32098834
- PMCID: PMC7043342
- DOI: 10.1128/mSystems.00025-20
SPD_1495 Contributes to Capsular Polysaccharide Synthesis and Virulence in Streptococcus pneumoniae
Abstract
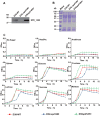
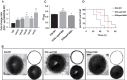
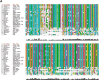
Streptococcus pneumoniae, a Gram-positive human pathogen, causes a series of serious diseases in humans. SPD_1495 from S. pneumoniae is annotated as a hypothetical ABC sugar-binding protein in the NCBI database, but there are few reports on detailed biological functions of SPD_1495. To fully study the influence of SPD_1495 on bacterial virulence in S. pneumoniae, we constructed a deletion mutant (D39Δspd1495) and an overexpressing strain (D39spd1495+). Comparative analysis of iTRAQ-based quantitative proteomic data of the wild-type D39 strain (D39-WT) and D39Δspd1495 showed that several differentially expressed proteins that participate in capsular polysaccharide synthesis, such as Cps2M, Cps2C, Cps2L, Cps2T, Cps2E, and Cps2D, were markedly upregulated in D39Δspd1495 Subsequent transmission electron microscopy and uronic acid detection assay confirmed that capsular polysaccharide synthesis was enhanced in D39Δspd1495 compared to that in D39-WT. Moreover, knockout of spd1495 resulted in increased capsular polysaccharide synthesis, as well as increased bacterial virulence, as confirmed by the animal study. Through a coimmunoprecipitation assay, surface plasmon resonance, and electrophoretic mobility shift assay, we found that SPD_1495 negatively regulated cps promoter expression by interacting with phosphorylated ComE, a negative transcriptional regulator for capsular polysaccharide formation. Overall, this study suggested that SPD_1495 negatively regulates capsular polysaccharide formation and thereby enhances bacterial virulence in the host. These findings also provide valuable insights into understanding the biology of this clinically important bacterium.IMPORTANCE Capsular polysaccharide is a key factor underlying the virulence of Streptococcus pneumoniae in human diseases. Thus, a deep understanding of capsular polysaccharide synthesis is essential for uncovering the pathogenesis of S. pneumoniae infection. In this study, we show that protein SPD_1495 interacts with phosphorylated ComE to negatively regulate the formation of capsular polysaccharide. Deletion of spd1495 increased capsular polysaccharide synthesis and thereby enhanced bacterial virulence. These findings further reveal the synthesis mechanism of capsular polysaccharide and provide new insight into the biology of this clinically important bacterium.
Keywords: Streptococcus pneumoniae; capsule; virulence.
Copyright © 2020 Zheng et al.
Figures



References
-
- World Health Organization. 2007. Pneumococcal conjugate vaccine for childhood immunization: WHO position paper. Wkly Epidemiol Rec 82:93–104. - PubMed
-
- O’Brien KL, Hib and Pneumococcal Global Burden of Disease Study Team, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi:10.1016/S0140-6736(09)61204-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
