microRNA-182 Negatively Influences the Neuroprotective Effect of Apelin Against Neuronal Injury in Epilepsy
- PMID: 32099369
- PMCID: PMC6996621
- DOI: 10.2147/NDT.S238826
microRNA-182 Negatively Influences the Neuroprotective Effect of Apelin Against Neuronal Injury in Epilepsy
Abstract
Purpose: To explore the neuroprotective effects and mechanisms of Apelin (APLN), and to study the regulation of APLN expression by microRNA (miRNA) in epilepsy.
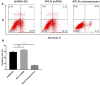
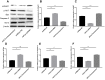
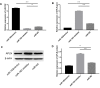
Materials and methods: In vitro and in vivo epileptic models were established with hippocampal neurons and Wistar rats. Apoptosis of neurons was identified by flow cytometry. Western blotting was used to detect the expression of proteins, and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was used to analyze the expression of miRNA and messenger RNA (mRNA). Bioinformatics software was used to predict target genes of miRNA, which were confirmed by dual-luciferase reporter gene system and functional experiments.
Results: Our study demonstrated protective effects of APLN against neuronal death in epilepsy both in vitro and in vivo. The underlying mechanisms involved are inhibiting the expression of metabotropic glutamate receptor 1 (mGluR1), Bax, and caspase-3; promoting the expression of Bcl-2; and increasing phosphorylated-AKT (p-AKT) levels in neurons. For the first time, we found that miR-182 could negatively regulate both transcriptional and translational levels of APLN, and that the up-regulation of miR-182 inhibited the expression of APLN and Bcl-2, and promoted the expression of Bax and caspase-3.
Conclusion: APLN could protect the neurons from injury in epilepsy by regulating the expression of apoptosis-associated proteins and mGluR1 and increasing p-AKT levels, which were attenuated by miR-182. Hence, miR-182/APLN may be potential targets for epilepsy control and treatment.
Keywords: apelin; epilepsy; miR-182; neuroprotective effects; regulation.
© 2020 Dong et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
MicroRNA-27a-3p Downregulation Inhibits Inflammatory Response and Hippocampal Neuronal Cell Apoptosis by Upregulating Mitogen-Activated Protein Kinase 4 (MAP2K4) Expression in Epilepsy: In Vivo and In Vitro Studies.Med Sci Monit. 2019 Nov 11;25:8499-8508. doi: 10.12659/MSM.916458. Med Sci Monit. 2019. PMID: 31710596 Free PMC article.
-
miR-21-5p protects hippocampal neurons of epileptic rats via inhibiting STAT3 expression.Adv Clin Exp Med. 2020 Jul;29(7):793-801. doi: 10.17219/acem/121929. Adv Clin Exp Med. 2020. PMID: 32745381
-
Knockdown of ZFAS1 Inhibits Hippocampal Neurons Apoptosis and Autophagy by Activating the PI3K/AKT Pathway via Up-regulating miR-421 in Epilepsy.Neurochem Res. 2020 Oct;45(10):2433-2441. doi: 10.1007/s11064-020-03103-1. Epub 2020 Jul 28. Neurochem Res. 2020. PMID: 32725369
-
Dysregulated microRNA-224/apelin axis associated with aggressive progression and poor prognosis in patients with prostate cancer.Hum Pathol. 2015 Feb;46(2):295-303. doi: 10.1016/j.humpath.2014.10.027. Epub 2014 Nov 26. Hum Pathol. 2015. PMID: 25532941
-
APLN promotes hepatocellular carcinoma through activating PI3K/Akt pathway and is a druggable target.Theranostics. 2019 Jul 9;9(18):5246-5260. doi: 10.7150/thno.34713. eCollection 2019. Theranostics. 2019. PMID: 31410213 Free PMC article.
Cited by
-
Targeting Adipokines: A Promising Therapeutic Strategy for Epilepsy.Neurochem Res. 2024 Nov;49(11):2973-2987. doi: 10.1007/s11064-024-04219-4. Epub 2024 Jul 26. Neurochem Res. 2024. PMID: 39060767 Review.
-
Neuroprotective effect of apelin-13 and other apelin forms-a review.Pharmacol Rep. 2024 Jun;76(3):439-451. doi: 10.1007/s43440-024-00587-4. Epub 2024 Apr 3. Pharmacol Rep. 2024. PMID: 38568371 Review.
-
The Apelinergic System: Apelin, ELABELA, and APJ Action on Cell Apoptosis: Anti-Apoptotic or Pro-Apoptotic Effect?Cells. 2022 Dec 30;12(1):150. doi: 10.3390/cells12010150. Cells. 2022. PMID: 36611944 Free PMC article. Review.
-
Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro.Brain Sci. 2022 Nov 8;12(11):1515. doi: 10.3390/brainsci12111515. Brain Sci. 2022. PMID: 36358441 Free PMC article.
-
Apelin/APJ: Another Player in the Cancer Biology Network.Int J Mol Sci. 2025 Mar 25;26(7):2986. doi: 10.3390/ijms26072986. Int J Mol Sci. 2025. PMID: 40243599 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

