Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options
- PMID: 32099464
- PMCID: PMC6996551
- DOI: 10.2147/CMAR.S202965
Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options
Abstract
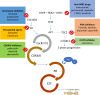
Neoadjuvant hormonal therapy (NEO-HT) is a possible treatment option for breast cancer (BC) patient with estrogen receptor positive (ER+) and HER2 negative (HER2-) disease. The absence of solid data on the type of drugs to be used and duration of treatment as well as lack of clear evidence of effectiveness of NEO-HT compared to chemotherapy (CT) reserve its use for patients with old age or frail conditions. However, the low pathologic complete response rate (pCR) obtained with tamoxifen or aromatase inhibitors (AIs) alone does not make NEO-HT as a suitable option for the neoadjuvant treatment of HR+ HER2-. The use of the cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors palbociclib, ribociclib and abemaciclib of the mammalian target of rapamycin (mTOR) inhibitor everolimus and of the phosphoinositide 3 kinase (PI3K) inhibitor taselisib together with endocrine therapy (ET) has become a standard in advanced breast cancer, showing clinical effectiveness and significantly prolonging median progression-free survival compared to ET only. In the early phase disease, the use of ET together with CDK 4/6, mTOR and PI3K inhibitors is still investigational. Data from recent studies are promising even though less impressive than in metastatic setting. In this context, the use of genomic-transcriptomic tools (such as ONCOTYPE, PAM50) and the identification of novel biomarkers (ESR1, PI3Kca, PDGF-R) on tissue or with liquid biopsy could help to select patient prone to respond to endocrine-combined therapy and able to achieve pCR. With our review, we aimed at evaluating the current state of the art in the treatment of locally advanced breast cancer with NEO-HT.
Keywords: CDK 4/6 inhibitors; PI3K inhibitors; aromatase inhibitors; breast cancer; mTOR inhibitors; neoadjuvant endocrine therapy.
© 2020 Grizzi et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. doi:10.1200/JCO.2005.04.005 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

