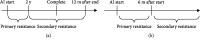High-Dose Toremifene as a Promising Candidate Therapy for Hormone Receptor-Positive Metastatic Breast Cancer with Secondary Resistance to Aromatase Inhibitors
- PMID: 32099680
- PMCID: PMC7037483
- DOI: 10.1155/2020/7156574
High-Dose Toremifene as a Promising Candidate Therapy for Hormone Receptor-Positive Metastatic Breast Cancer with Secondary Resistance to Aromatase Inhibitors
Abstract
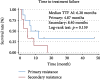
There are currently no established second- and later-line therapies for postmenopausal women with hormone receptor-positive advanced or metastatic breast cancer. We examined the efficacy of high-dose toremifene (HD-TOR) for this patient group and whether aromatase inhibitor (AI) resistance influences HD-TOR treatment outcome. This retrospective analysis investigated the outcomes of 19 women with postmenopausal hormone-sensitive recurrent or metastatic breast cancer who received HD-TOR, defined as 120 mg daily from 2012 to 2016. The median follow-up duration was 9.67 months. The overall response rate (ORR) and clinical benefit rate (CBR) were compared between various clinical subgroups, including patients exhibiting primary or secondary AI resistance as defined by the timing of recurrence or progression. Time to treatment failure (TTF) was estimated by the Kaplan-Meier method and compared between subgroups by the log-rank test. The overall ORR was 21.1%, and the CBR was 31.6%. CBR was significantly higher for patients without liver metastasis (50% vs. 0%, p = 0.044). Nine cases exhibited primary and eight cases secondary AI resistance. Both ORR and CBR were higher in patients with secondary AI resistance (25% vs. 0%, p = 0.087; 38% vs. 11%, p = 0.29). The median TTF was 6.2 months in the entire AI-resistant group (n = 17) and was longer in the secondary resistance subgroup than in the primary resistance subgroup (8.40 vs. 4.87 months; log-rank: p = 0.159). High-dose TOR appears to be most effective for postmenopausal breast cancer cases with secondary resistance to AIs, cases without prior AI treatment, and cases without liver metastasis. The detailed mechanisms of AI resistance and the clinical features of responsive cases need to be further clarified to identify the best candidates for HD-TOR.
Copyright © 2020 Atsushi Fushimi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Hortobagyi G. N., Stemmer S. M., Burris H. A., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Annals of Oncology. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous