Exploratory Evaluation of Bezlotoxumab on Outcomes Associated With Clostridioides difficile Infection in MODIFY I/II Participants With Cancer
- PMID: 32099847
- PMCID: PMC7029680
- DOI: 10.1093/ofid/ofaa038
Exploratory Evaluation of Bezlotoxumab on Outcomes Associated With Clostridioides difficile Infection in MODIFY I/II Participants With Cancer
Abstract
Background: The incidence of Clostridioides difficile infection (CDI) is reportedly higher and the cure rate lower in individuals with cancer vs those without cancer. An exploratory post hoc analysis of the MODIFY I/II trials (NCT01241552/NCT01513239) investigated how bezlotoxumab affected the rate of CDI-related outcomes in participants with cancer.
Methods: Participants received a single infusion of bezlotoxumab (10 mg/kg) or placebo during anti-CDI antibacterial treatment. A post hoc analysis of CDI-related outcomes was conducted in subgroups of MODIFY I/II participants with and without cancer.
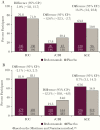
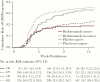
Results: Of 1554 participants in the modified intent-to-treat (mITT) population, 382 (24.6%) were diagnosed with cancer (bezlotoxumab 190, placebo 192). Of participants without cancer, 591 and 581 received bezlotoxumab and placebo, respectively. In the placebo group, initial clinical cure (ICC) was achieved by fewer cancer participants vs participants without cancer (71.9% vs 83.1%; absolute difference, -11.3%; 95% CI, -18.6% to -4.5%); however, CDI recurrence (rCDI) rates were similar in cancer (30.4%) and noncancer (34.0%) participants. In participants with cancer, bezlotoxumab treatment had no effect on ICC rate compared with placebo (76.8% vs 71.9%), but resulted in a statistically significant reduction in rCDI vs placebo (17.8% vs 30.4%; absolute difference, -12.6%; 95% CI, -22.5% to -2.7%).
Conclusions: In this post hoc analysis of participants with cancer enrolled in MODIFY I/II, the rate of rCDI in bezlotoxumab-treated participants was lower than in placebo-treated participants. Additional studies are needed to confirm these results.
Clinical trial registration: MODIFY I (NCT01241552), MODIFY II (NCT01513239).
Keywords: CDI recurrence; Clostridioides difficile; cancer; hematologic malignancy; solid tumor.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- European Centre for Disease Prevention and Control. Surveillance report: point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012. Available at: http://ecdc.europa.eu/en/publications/publications/healthcare-associated.... Accessed 08 January 2020.
-
- Gravel D, Miller M, Simor A, et al. ; Canadian Nosocomial Infection Surveillance Program Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis 2009; 48:568–76. - PubMed
-
- Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. - PubMed
-
- Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

