Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy
- PMID: 32099991
- PMCID: PMC7713693
- DOI: 10.1093/cid/ciaa177
Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy
Abstract
Background: Treatment initiation with integrase strand transfer inhibitors (INSTIs) has been associated with excess weight gain. Whether similar gains are seen after switch to INSTIs among virologically suppressed persons is less clear. We assessed pre/post-INSTI weight changes from AIDS Clinical Trials Group participants (A5001 and A5322).
Methods: Participants who were in follow-up from 1997-2017 and switched to INSTI-based antiretroviral regimens were included. Piecewise linear mixed-effects models adjusting for age, sex, race/ethnicity, baseline BMI, nadir and current CD4+ T-cell count, smoking, diabetes and follow-up time with suppressed HIV-1 RNA examined weight and waist circumference change before and after first switch to INSTIs. Linear spline models with a single knot at time of switch accounted for nonlinear trends.
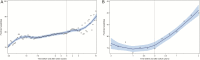
Results: The 972 participants who switched to INSTIs were 81% male and 50% nonwhite with a median age at switch of 50 years, CD4+ T-cell count 512 cells/μL, and BMI 26.4 kg/m2. Restricting to persons with suppressed HIV-1 RNA at switch (n = 691), women, blacks, and persons ≥60 years experienced greater weight gain in the 2 years after versus before switch. In adjusted models, white or black race, age ≥60, and BMI ≥30 kg/m2 at switch were associated with greater weight gain following switch among women; age ≥60 was the greatest risk factor among men. Trends for waist circumference were similar.
Conclusions: Yearly weight gain increased following switch to INSTIs, particularly for women, blacks, and persons aged ≥60. Concomitant increases in waist circumference suggest that this weight gain is associated with an increase in fat mass.
Keywords: HIV dolutegravir; integrase inhibitor; weight gain; women.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. - PubMed
-
- Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS 2020; 34:109–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

