Pregnancy outcomes in interferon-beta-exposed patients with multiple sclerosis: results from the European Interferon-beta Pregnancy Registry
- PMID: 32100126
- PMCID: PMC7293672
- DOI: 10.1007/s00415-020-09762-y
Pregnancy outcomes in interferon-beta-exposed patients with multiple sclerosis: results from the European Interferon-beta Pregnancy Registry
Abstract
Background: Family planning is an important consideration for women with multiple sclerosis (MS), who are often diagnosed during their reproductive years. Currently, limited data are available on pregnancy outcomes in patients exposed to interferon-beta (IFN-beta) before or during pregnancy. Here, we present the cumulative pregnancy exposure data and prevalence of pregnancy and infant outcomes in IFN-beta-exposed pregnant women with MS from the European IFN-beta Pregnancy Registry.
Methods: Using spontaneous and solicited reports, the registry collected data from 26 countries of the European Economic Area, consisting of information on women with MS identifying themselves to one of the Marketing Authorisation Holders (Bayer, Biogen, Merck KGaA, and Novartis) or healthcare professionals as pregnant and exposed to IFN-beta during pregnancy or within 1 month before conception. The outcomes collected by the registry included ectopic pregnancies, spontaneous abortions, elective terminations, live, and stillbirths with or without congenital anomalies. The prevalence of pregnancy outcomes was put in context with those reported in the general population.
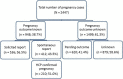
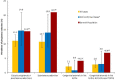
Results: Between 2009 and 2017, the registry collected 948 pregnancy reports with a known pregnancy outcome. Overall, 82.0% (777/948) of pregnancies resulted in live birth without congenital anomaly. When comparing IFN-beta-exposed pregnancies with the general population, the prevalence of spontaneous abortions (10.7% vs. 10-21%) and congenital anomalies in live births (2.1% vs. 2.1-4.1%) were found to be within reported ranges.
Conclusions: The data gathered from these pregnancy cases suggest no evidence that IFN-beta exposure before conception and/or during pregnancy adversely increases the rate of congenital anomalies or spontaneous abortions.
Keywords: Congenital anomalies; Disease-modifying drugs; Interferon-beta; Multiple sclerosis; Pregnancy; Spontaneous abortions.
Conflict of interest statement
KH has received honoraria and research support from Bayer, Biogen, Teva, Novartis, Sanofi Genzyme, and Merck KGaA. YG is an employee of Novartis Pharma AG. MS is an employee of Merck Healthcare KGaA, Darmstadt, Germany. CP is an employee of Biogen. AA is an employee of Bayer AG. JK is an employee of SynteractHCR GmbH. PH has received honoraria and research support from Bayer, Biogen, Novartis, and Merck KGaA, and is a member of the European Interferon-beta Pregnancy Study Group. AO has nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

