Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis
- PMID: 32100144
- PMCID: PMC7103014
- DOI: 10.1007/s10549-020-05577-7
Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis
Abstract
Purpose: In the absence of head-to-head trial data, network meta-analysis (NMA) was used to compare trastuzumab emtansine (T-DM1) with other approved treatments for previously treated patients with unresectable or metastatic HER2-positive breast cancer (BC).
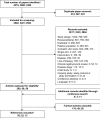
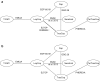
Methods: Systematic reviews were conducted of published controlled trials of treatments for unresectable or metastatic HER2-positive BC with early relapse (≤ 6 months) following adjuvant therapy or progression after trastuzumab (Tras) + taxane published from January 1998 to January 2018. Random-effects NMA was conducted for overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and safety endpoints.
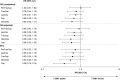
Results: The NMA included regimens from seven randomized controlled trials: T-DM1 and combinations of Tras, capecitabine (Cap), lapatinib (Lap), neratinib, or pertuzumab (Per; unapproved). OS results favored T-DM1 over approved comparators: hazard ratio (HR) (95% credible interval [95% CrI]) vs Cap 0.68 (0.39, 1.10), LapCap 0.76 (0.51, 1.07), TrasCap 0.78 (0.44, 1.19). PFS trends favored T-DM1 over all other treatments: HR (95% CrI) vs Cap 0.38 (0.19, 0.74), LapCap 0.65 (0.40, 1.10), TrasCap 0.62 (0.34, 1.18); ORR with T-DM1 was more favorable than with all approved treatments. In surface under cumulative ranking curve (SUCRA) analysis T-DM1 ranked highest for all efficacy outcomes. Discontinuation due to adverse events was less likely with T-DM1 than with all comparators except neratinib. In general, gastrointestinal side effects were less likely and elevated liver transaminases and thrombocytopenia more likely with T-DM1 than with comparators.
Conclusions: The efficacy and tolerability profiles of T-DM1 are generally favorable compared with other treatments for unresectable or metastatic HER2-positive BC.
Keywords: Capecitabine; Lapatinib; Locally advanced; Neratinib; Pertuzumab; Trastuzumab emtansine.
Conflict of interest statement
NP is an employee of F. Hoffmann-La Roche AG and owns F. Hoffmann-La Roche AG stock. AR is an employee of F. Hoffmann-La Roche AG. VD Consultant/Advisory role: AbbVie, Astellas, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Nektar, Novartis, Odonate, Pfizer, Roche/Genentech, Seattle Genetics, Tesaro. IK Consultant/Advisory role: Bristol-Myers Squibb, Context Therapeutics, Daiichi Sankyo, MacroGenics, Merck, Novartis, Roche/Genentech, Seattle Genetics, Taiho Oncology. XP has nothing to declare. AU has received travel support from Roche
Figures


Similar articles
-
Trastuzumab-emtansine versus other anti-HER2 regimens in early or unresectable or metastatic HER-2 positive breast cancer: systematic review and network meta-analysis.Rev Peru Med Exp Salud Publica. 2024 May 27;41(1):7-18. doi: 10.17843/rpmesp.2024.411.13351. Rev Peru Med Exp Salud Publica. 2024. PMID: 38808848 Free PMC article.
-
A network meta-analysis on the efficacy of HER2-targeted agents in combination with taxane-containing regimens for treatment of HER2-positive metastatic breast cancer.Breast Cancer. 2020 Mar;27(2):186-196. doi: 10.1007/s12282-019-01007-9. Epub 2019 Sep 16. Breast Cancer. 2020. PMID: 31529262
-
Efficacy and Safety of HER2-Targeted Agents for Breast Cancer with HER2-Overexpression: A Network Meta-Analysis.PLoS One. 2015 May 20;10(5):e0127404. doi: 10.1371/journal.pone.0127404. eCollection 2015. PLoS One. 2015. PMID: 25993646 Free PMC article.
-
Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline.J Clin Oncol. 2014 Jul 1;32(19):2078-99. doi: 10.1200/JCO.2013.54.0948. Epub 2014 May 5. J Clin Oncol. 2014. PMID: 24799465 Free PMC article.
-
Lapatinib and trastuzumab in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptor-positive breast cancer which over-expresses human epidermal growth factor 2 (HER2): a systematic review and economic analysis.Health Technol Assess. 2011;15(42):1-93, iii-iv. doi: 10.3310/hta15420. Health Technol Assess. 2011. PMID: 22152751 Free PMC article.
Cited by
-
Drug resistance gene expression and chemotherapy sensitivity detection in Chinese women with different molecular subtypes of breast cancer.Cancer Biol Med. 2020 Nov 15;17(4):1014-1025. doi: 10.20892/j.issn.2095-3941.2020.0157. Epub 2020 Dec 15. Cancer Biol Med. 2020. PMID: 33299650 Free PMC article.
-
Quantitative chemometric phenotyping of three-dimensional liver organoids by Raman spectral imaging.Cell Rep Methods. 2023 Mar 31;3(4):100440. doi: 10.1016/j.crmeth.2023.100440. eCollection 2023 Apr 24. Cell Rep Methods. 2023. PMID: 37159662 Free PMC article.
-
Invasive ductal breast carcinoma preceded by CALR-positive essential thrombocythemia.Clin Case Rep. 2021 Feb 9;9(3):1732-1736. doi: 10.1002/ccr3.3892. eCollection 2021 Mar. Clin Case Rep. 2021. PMID: 33768925 Free PMC article.
-
Enhanced brain delivery and therapeutic activity of trastuzumab after blood-brain barrier opening by NEO100 in mouse models of brain-metastatic breast cancer.Neuro Oncol. 2021 Oct 1;23(10):1656-1667. doi: 10.1093/neuonc/noab041. Neuro Oncol. 2021. PMID: 33659980 Free PMC article.
-
Model-based assessment of combination therapies - ranking of radiosensitizing agents in oncology.BMC Cancer. 2023 May 6;23(1):409. doi: 10.1186/s12885-023-10899-y. BMC Cancer. 2023. PMID: 37149596 Free PMC article.
References
-
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Physicians (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. 10.1200/JCO.2013.50.9984 - PubMed
-
- Roche_Pharma_AG (2018) Herceptin® summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/herceptin-epa.... Accessed 01 Apr 2019
-
- Genentech_Inc (2010) HERCEPTIN prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.... Accessed 01 Apr 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

