Aloin Protects Against Blood-Brain Barrier Damage After Traumatic Brain Injury in Mice
- PMID: 32100248
- PMCID: PMC7270422
- DOI: 10.1007/s12264-020-00471-0
Aloin Protects Against Blood-Brain Barrier Damage After Traumatic Brain Injury in Mice
Abstract
Aloin is a small-molecule drug well known for its protective actions in various models of damage. Traumatic brain injury (TBI)-induced cerebral edema from secondary damage caused by disruption of the blood-brain barrier (BBB) often leads to an adverse prognosis. Since the role of aloin in maintaining the integrity of the BBB after TBI remains unclear, we explored the protective effects of aloin on the BBB using in vivo and in vitro TBI models. Adult male C57BL/6 mice underwent controlled cortical impact injury, and mouse brain capillary endothelial bEnd.3 cells underwent biaxial stretch injury, then both received aloin treatment. In the animal experiments, we found 20 mg/kg aloin to be the optimum concentration to decrease cerebral edema, decrease disruption of the BBB, and improve neurobehavioral performance after cortical impact injury. In the cellular studies, the optimum concentration of 40 μg/mL aloin reduced apoptosis and reversed the loss of tight junctions by reducing the reactive oxygen species levels and changes in mitochondrial membrane potential after stretch injury. The mechanisms may be that aloin downregulates the phosphorylation of p38 mitogen-activated protein kinase, the activation of p65 nuclear factor-kappa B, and the ratios of B cell lymphoma (Bcl)-2-associated X protein/Bcl-2 and cleaved caspase-3/caspase-3. We conclude that aloin exhibits these protective effects on the BBB after TBI through its anti-oxidative stress and anti-apoptotic properties in mouse brain capillary endothelial cells. Aloin may thus be a promising therapeutic drug for TBI.
Keywords: Aloin; Apoptosis; Blood–brain barrier; Oxidative stress; Traumatic brain injury.
Conflict of interest statement
The authors declare no competing interests.
Figures



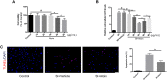




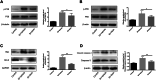
References
-
- Needham EJ, Helmy A, Zanier ER, Jones JL, Coles AJ, Menon DK. The immunological response to traumatic brain injury. J Neuroimmunol. 2019;332:112–125. - PubMed
-
- Yang DX, Jing Y, Liu YL, Xu ZM, Yuan F, Wang ML, et al. Inhibition of transient receptor potential vanilloid 1 attenuates blood–brain barrier disruption after traumatic brain injury in mice. J Neurotrauma. 2019;36:1279–1290. - PubMed
-
- Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, et al. Traumatic brain injury in China. Lancet Neurol. 2019;18:286–295. - PubMed
-
- Liu YL, Yuan F, Yang DX, Xu ZM, Jing Y, Yang GY, et al. Adjudin attenuates cerebral edema and improves neurological function in mice with experimental traumatic brain injury. J Neurotrauma. 2018;35:2850–2860. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

