Long non-coding RNAs in development and disease: conservation to mechanisms
- PMID: 32100288
- PMCID: PMC8638664
- DOI: 10.1002/path.5405
Long non-coding RNAs in development and disease: conservation to mechanisms
Abstract
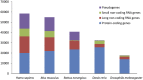
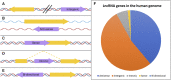
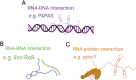
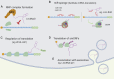
Our genomes contain the blueprint of what makes us human and many indications as to why we develop disease. Until the last 10 years, most studies had focussed on protein-coding genes, more specifically DNA sequences coding for proteins. However, this represents less than 5% of our genomes. The other 95% is referred to as the 'dark matter' of our genomes, our understanding of which is extremely limited. Part of this 'dark matter' includes regions that give rise to RNAs that do not code for proteins. A subset of these non-coding RNAs are long non-coding RNAs (lncRNAs), which in particular are beginning to be dissected and their importance to human health revealed. To improve our understanding and treatment of disease it is vital that we understand the molecular and cellular function of lncRNAs, and how their misregulation can contribute to disease. It is not yet clear what proportion of lncRNAs is actually functional; conservation during evolution is being used to understand the biological importance of lncRNA. Here, we present key themes within the field of lncRNAs, emphasising the importance of their roles in both the nucleus and the cytoplasm of cells, as well as patterns in their modes of action. We discuss their potential functions in development and disease using examples where we have the greatest understanding. Finally, we emphasise why lncRNAs can serve as biomarkers and discuss their emerging potential for therapy. © 2020 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: X chromosome inactivation; anti-sense lncRNAs; cancer; conservation; development; diabetes; long intergenic non-coding RNA; long non-coding RNA; neurodegenerative disease; stem cells; translation.
© 2020 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures



References
-
- Brown CJ, Ballabio A, Rupert JL, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 1991; 349 : 38–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

