Sequential boost of intensity-modulated radiotherapy with chemotherapy for inoperable esophageal squamous cell carcinoma: A prospective phase II study
- PMID: 32100452
- PMCID: PMC7163105
- DOI: 10.1002/cam4.2933
Sequential boost of intensity-modulated radiotherapy with chemotherapy for inoperable esophageal squamous cell carcinoma: A prospective phase II study
Abstract
Purpose: This prospective phase II study aimed to determine the efficacy and tolerability of sequential boost of intensity-modulated radiation therapy (IMRT) with chemotherapy for patients with inoperable esophageal squamous cell carcinoma (ESCC).
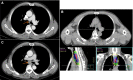
Methods: Patients with histologically or cytologically proven inoperable ESCC were enrolled in this study (ChiCTR-OIC-17010485). A larger target volume for subclinical lesion was irradiated with 50 Gy, and then, a smaller target volume only including gross tumor was boosted to 66 Gy. The fraction dose was 2 Gy, and no elective node was irradiated. Concurrent and consolidation chemotherapy of fluorouracil (600 mg/m2 , days 1-3) plus cisplatin (25 mg/m2 , days 1-3) was administered every 4 weeks, for 4 cycles in total. The primary endpoint was 2-year progression-free survival (PFS).
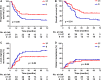
Results: Eighty-eight patients were enrolled in this study. The median age was 65 years (range: 45-75 years), and 69 patients (78.4%) were men. With the median follow-up of 26 (range: 3-95) months, the 2- and 5-year PFS were 39.3% and 36.9%, respectively, and overall survival (OS) were 57.1% and 39.2%, respectively. Tumor stage and concurrent chemotherapy were independent OS predictors. Major acute adverse events were myelosuppression and esophagitis, most of which were grades 1-2. Nine percent and 2.3% of patients had grade 3 acute esophagitis and late esophageal strictures, respectively.
Conclusions: Sequential boost to 66 Gy by IMRT with chemotherapy was safe and effective for inoperable ESCC. A randomized phase III study to compare with standard dose of 50 Gy is warranted.
Keywords: chemoradiotherapy; esophageal squamous cell carcinoma; intensity-modulated radiotherapy.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
No authors declare conflicts of interest.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo‐adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20:231‐238. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9‐29. - PubMed
-
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400‐412. - PubMed
-
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long‐term follow‐up of a prospective randomized trial (RTOG 85–01) radiation therapy oncology group. JAMA. 1999;281:1623‐1627. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

