Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration
- PMID: 32100541
- PMCID: PMC7146758
- DOI: 10.1021/acsami.9b23100
Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration
Abstract
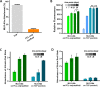
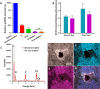
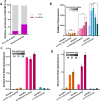
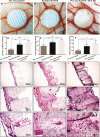
Tissue engineering (TE)-based bone grafts are favorable alternatives to autografts and allografts. Both biochemical properties and the architectural features of TE scaffolds are crucial in their design process. Synthetic polymers are attractive biomaterials to be used in the manufacturing of TE scaffolds, due to various advantages, such as being relatively inexpensive, enabling precise reproducibility, possessing tunable mechanical/chemical properties, and ease of processing. However, such scaffolds need modifications to improve their limited interaction with biological tissues. Structurally, multiscale porosity is advantageous over single-scale porosity; therefore, in this study, we have considered two key points in the design of a bone repair material; (i) manufacture of multiscale porous scaffolds made of photocurable polycaprolactone (PCL) by a combination of emulsion templating and three-dimensional (3D) printing and (ii) decoration of these scaffolds with the in vitro generated bone-like extracellular matrix (ECM) to create biohybrid scaffolds that have improved biological performance compared to PCL-only scaffolds. Multiscale porous scaffolds were fabricated, bone cells were cultured on them, and then they were decellularized. The biological performance of these constructs was tested in vitro and in vivo. Mesenchymal progenitors were seeded on PCL-only and biohybrid scaffolds. Cells not only showed improved attachment on biohybrid scaffolds but also exhibited a significantly higher rate of cell growth and osteogenic activity. The chick chorioallantoic membrane (CAM) assay was used to explore the angiogenic potential of the biohybrid scaffolds. The CAM assay indicated that the presence of the in vitro generated ECM on polymeric scaffolds resulted in higher angiogenic potential and a high degree of tissue infiltration. This study demonstrated that multiscale porous biohybrid scaffolds present a promising approach to improve bioactivity, encourage precursors to differentiate into mature bones, and to induce angiogenesis.
Keywords: 3D printing; angiogenesis; biohybrid; decellularization; emulsion templating; polyHIPE; tissue engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures