Influence of Glucose Concentration on Colony-Forming Efficiency and Biological Performance of Primary Human Tissue-Derived Progenitor Cells
- PMID: 32100548
- PMCID: PMC8804831
- DOI: 10.1177/1947603520906605
Influence of Glucose Concentration on Colony-Forming Efficiency and Biological Performance of Primary Human Tissue-Derived Progenitor Cells
Abstract
Objective: Glucose concentrations used in current cell culture methods are a significant departure from physiological glucose levels. The study focuses on comparing the effects of glucose concentrations on primary human progenitors (connective tissue progenitors [CTPs]) used for cartilage repair.
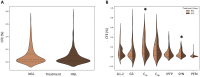
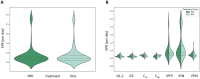
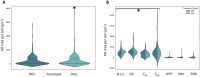
Design: Cartilage- (Outerbridge grade 1, 2, 3; superficial and deep zone cartilage), infrapatellar fatpad-, synovium-, and periosteum-derived cells were obtained from 63 patients undergoing total knee arthroplasty and cultured simultaneously in fresh chondrogenic media containing 25 mM glucose (HGL) or 5 mM glucose (NGL) for pairwise comparison. Automated ASTM-based quantitative image analysis was used to determine colony-forming efficiency (CFE), effective proliferation rates (EPR), and sulfated-proteoglycan (GAG-ECM) staining of the CTPs across tissue sources.
Results: HGL resulted in increased cell cultures with CFE = 0 compared with NGL in all tissue sources (P = 0.049). The CFE in NGL was higher than HGL for superficial cartilage (P < 0.001), and contrary for synovium-derived CTPs (P = 0.046) when CFE > 0. EPR of the CTPs did not differ between the media in the 6-day assay time period (P = 0.082). The GAG-ECM area of the CTPs and their progeny was increased in presence of HGL (P = 0.027).
Conclusion: Glucose concentration is critical to progenitor's physiology and should be taken into account in the setting of protocols for clinical or in vitro cell expansion strategies.
Keywords: cartilage; cell therapy; colony-forming efficiency; glucose; stem and progenitors.
Conflict of interest statement
Figures

Similar articles
-
Characterization of heterogeneous primary human cartilage-derived cell population using non-invasive live-cell phase-contrast time-lapse imaging.Cytotherapy. 2021 Jun;23(6):488-499. doi: 10.1016/j.jcyt.2020.09.006. Epub 2020 Oct 20. Cytotherapy. 2021. PMID: 33092987 Free PMC article.
-
Native-Osteoarthritic Joint Resident Stem and Progenitor Cells for Cartilage Cell-Based Therapies: A Quantitative Comparison With Respect to Concentration and Biological Performance.Am J Sports Med. 2019 Dec;47(14):3521-3530. doi: 10.1177/0363546519880905. Epub 2019 Oct 31. Am J Sports Med. 2019. PMID: 31671273
-
Donor-matched comparison of chondrogenic progenitors resident in human infrapatellar fat pad, synovium, and periosteum - implications for cartilage repair.Connect Tissue Res. 2019 Nov;60(6):597-610. doi: 10.1080/03008207.2019.1611795. Epub 2019 May 14. Connect Tissue Res. 2019. PMID: 31020864
-
The Potential for Synovium-derived Stem Cells in Cartilage Repair.Curr Stem Cell Res Ther. 2018 Feb 23;13(3):174-184. doi: 10.2174/1574888X12666171002111026. Curr Stem Cell Res Ther. 2018. PMID: 28969580 Review.
-
Engineering principles of clinical cell-based tissue engineering.J Bone Joint Surg Am. 2004 Jul;86(7):1541-58. doi: 10.2106/00004623-200407000-00029. J Bone Joint Surg Am. 2004. PMID: 15252108 Review.
Cited by
-
Assessment of Clinical, Tissue, and Cell-Level Metrics Identify Four Biologically Distinct Knee Osteoarthritis Patient Phenotypes.Cartilage. 2022 Jan-Mar;13(1):19476035221074003. doi: 10.1177/19476035221074003. Cartilage. 2022. PMID: 35109693 Free PMC article.
-
Metabolic Priming as a Tool in Redox and Mitochondrial Theragnostics.Antioxidants (Basel). 2023 May 10;12(5):1072. doi: 10.3390/antiox12051072. Antioxidants (Basel). 2023. PMID: 37237939 Free PMC article. Review.
-
Characterization of heterogeneous primary human cartilage-derived cell population using non-invasive live-cell phase-contrast time-lapse imaging.Cytotherapy. 2021 Jun;23(6):488-499. doi: 10.1016/j.jcyt.2020.09.006. Epub 2020 Oct 20. Cytotherapy. 2021. PMID: 33092987 Free PMC article.
-
From regeneration to osteoarthritis in the knee joint: The role shift of cartilage-derived progenitor cells.Front Cell Dev Biol. 2022 Oct 20;10:1010818. doi: 10.3389/fcell.2022.1010818. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36340024 Free PMC article. Review.
-
Effect of glucose depletion and fructose administration during chondrogenic commitment in human bone marrow-derived stem cells.Stem Cell Res Ther. 2022 Dec 27;13(1):533. doi: 10.1186/s13287-022-03214-2. Stem Cell Res Ther. 2022. PMID: 36575539 Free PMC article.
References
-
- Mantripragada VP, Piuzzi NS, Bova WA, Boehm C, Obuchowski NA, Lefebvre V, et al.. Donor-matched comparison of chondrogenic progenitors resident in human infrapatellar fat pad, synovium and periosteum and correlation to patient age and gender—implications for cartilage repair. Connect Tissue Res. 2019;60(6):597-601. - PubMed
-
- Mantripragada VP, Bova W, Boehm C, Piuzzi NS, Obuchowski NA, Midura RJ, et al.. Primary cells isolated from human knee cartilage reveal decreased prevalence of progenitor cells but comparable biological potential during osteoarthritic disease progression. J Bone Joint Surg Am. 2018;100(20):1771-80. - PMC - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al.. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

