Differentiation of lymphocytic-plasmacytic enteropathy and small cell lymphoma in cats using histology-guided mass spectrometry
- PMID: 32100916
- PMCID: PMC7096630
- DOI: 10.1111/jvim.15742
Differentiation of lymphocytic-plasmacytic enteropathy and small cell lymphoma in cats using histology-guided mass spectrometry
Abstract
Background: Differentiation of lymphocytic-plasmacytic enteropathy (LPE) from small cell lymphoma (SCL) in cats can be challenging.
Hypothesis/objective: Histology-guided mass spectrometry (HGMS) is a suitable method for the differentiation of LPE from SCL in cats.
Animals: Forty-one cats with LPE and 52 cats with SCL.
Methods: This is a retrospective clinicopathologic study. Duodenal tissue samples of 17 cats with LPE and 22 cats with SCL were subjected to HGMS, and the acquired data were used to develop a linear discriminate analysis (LDA) machine learning algorithm. The algorithm was subsequently validated using a separate set of 24 cats with LPE and 30 cats with SCL. Cases were classified as LPE or SCL based on a consensus by an expert panel consisting of 5-7 board-certified veterinary specialists. Histopathology, immunohistochemistry, and clonality testing were available for all cats. The panel consensus classification served as a reference for the calculation of test performance parameters.
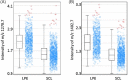
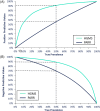
Results: Relative sensitivity, specificity, and accuracy of HGMS were 86.7% (95% confidence interval [CI]: 74.5%-98.8%), 91.7% (95% CI: 80.6%-100%), and 88.9% (95% CI: 80.5%-97.3%), respectively. Comparatively, the clonality testing had a sensitivity, specificity, and accuracy of 85.7% (95% CI: 72.8%-98.7%), 33.3% (95% CI: 14.5%-52.2%), and 61.5% (95% CI: 48.3%-74.8%) relative to the panel decision.
Conclusions and clinical importance: Histology-guided mass spectrometry was a reliable technique for the differentiation of LPE from SCL in duodenal formalin-fixed paraffin-embedded samples of cats and might have advantages over tests currently considered state of the art.
Keywords: HGMS; MALDI mass spectrometry; PARR; PCR for antigen receptor rearrangements; chronic enteropathy; clonality testing; feline; inflammatory bowel disease; inflammatory enteropathy; small cell lymphoma.
© 2020 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Marsilio, Lidbury, Suchodolski, and Steiner are or were employed by the Gastrointestinal Laboratory at Texas A&M University, at the time of the study, which offers laboratory testing, including histopathology services, on a fee‐for‐service basis. Smoot, Seeley, and Powell are or were employed by New River VDL, LLC at the time of study. New River VDL, LLC offers a commercially available histology guided mass spectrometry assay for cats on a fee‐for‐service basis. Steiner serves as a paid consultant for New River VDL. Estep is employed by Texas Veterinary Pathology, LLC, which offers histopathology for animals on a fee‐for‐service basis. Newman, Warry, Flory, Giaretta, and Morley have no conflicts to disclose.
Figures


Similar articles
-
Characterization of the intestinal mucosal proteome in cats with inflammatory bowel disease and alimentary small cell lymphoma.J Vet Intern Med. 2021 Jan;35(1):179-189. doi: 10.1111/jvim.16003. Epub 2021 Jan 20. J Vet Intern Med. 2021. PMID: 33471936 Free PMC article.
-
Results of histopathology, immunohistochemistry, and molecular clonality testing of small intestinal biopsy specimens from clinically healthy client-owned cats.J Vet Intern Med. 2019 Mar;33(2):551-558. doi: 10.1111/jvim.15455. Epub 2019 Feb 28. J Vet Intern Med. 2019. PMID: 30820999 Free PMC article.
-
Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis.J Vet Intern Med. 2021 Nov;35(6):2673-2684. doi: 10.1111/jvim.16231. Epub 2021 Aug 10. J Vet Intern Med. 2021. PMID: 34374109 Free PMC article.
-
Differentiating Inflammatory Bowel Disease from Alimentary Lymphoma in Cats: Does It Matter?Vet Clin North Am Small Anim Pract. 2021 Jan;51(1):93-109. doi: 10.1016/j.cvsm.2020.09.009. Vet Clin North Am Small Anim Pract. 2021. PMID: 33187624 Review.
-
Feline chronic enteropathy.J Small Anim Pract. 2021 Jun;62(6):409-419. doi: 10.1111/jsap.13332. Epub 2021 Apr 5. J Small Anim Pract. 2021. PMID: 33821508 Review.
Cited by
-
Chronic Inflammatory Enteropathy and Low-Grade Intestinal T-Cell Lymphoma Are Associated with Altered Microbial Tryptophan Catabolism in Cats.Animals (Basel). 2023 Dec 23;14(1):67. doi: 10.3390/ani14010067. Animals (Basel). 2023. PMID: 38200798 Free PMC article.
-
Dysbiosis index to evaluate the fecal microbiota in healthy cats and cats with chronic enteropathies.J Feline Med Surg. 2022 Jun;24(6):e1-e12. doi: 10.1177/1098612X221077876. Epub 2022 Mar 10. J Feline Med Surg. 2022. PMID: 35266809 Free PMC article.
-
Characterization of the intestinal mucosal proteome in cats with inflammatory bowel disease and alimentary small cell lymphoma.J Vet Intern Med. 2021 Jan;35(1):179-189. doi: 10.1111/jvim.16003. Epub 2021 Jan 20. J Vet Intern Med. 2021. PMID: 33471936 Free PMC article.
-
Multimodal Imaging Mass Spectrometry: Next Generation Molecular Mapping in Biology and Medicine.J Am Soc Mass Spectrom. 2020 Dec 2;31(12):2401-2415. doi: 10.1021/jasms.0c00232. Epub 2020 Sep 4. J Am Soc Mass Spectrom. 2020. PMID: 32886506 Free PMC article. Review.
-
Intestinal S100/Calgranulin Expression in Cats with Chronic Inflammatory Enteropathy and Intestinal Lymphoma.Animals (Basel). 2022 Aug 11;12(16):2044. doi: 10.3390/ani12162044. Animals (Basel). 2022. PMID: 36009635 Free PMC article.
References
-
- Richter KP. Feline gastrointestinal lymphoma. Vet Clin North Am Small Anim Pract. 2003;33:1083‐1098. vii. - PubMed
-
- Sabattini S, Bottero E, Turba ME, Vicchi F, Bo S, Bettini G. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J Small Anim Pract. 2016;57:396‐401. - PubMed
-
- Moore PF, Rodriguez‐Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658‐668. - PubMed
-
- Keller SM, Vernau W, Moore PF. Clonality testing in veterinary medicine: a review with diagnostic guidelines. Vet Pathol. 2016;53:711‐725. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

