Structural Effect on Adjuvanticity of Saponins
- PMID: 32101001
- PMCID: PMC8832471
- DOI: 10.1021/acs.jmedchem.9b02063
Structural Effect on Adjuvanticity of Saponins
Abstract
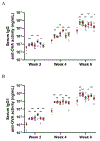
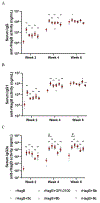
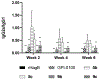
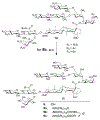
We have prepared a number of saponin-based vaccine adjuvant candidates. These unnatural saponins have a different terminal-functionalized side chain incorporated into the glucuronic acid unit that is attached to a triterpenoid core at its C3 position. The semisynthetic saponin adjuvants have shown significantly different immunostimulatory activities, suggesting that the structure of the side chain, triterpenoid core, and oligosaccharide domain together orchestrate saponin adjuvant's potentiation of immune responses. Among these new adjuvant candidates, VSA-2 (5b), a derivative of Momordica saponin (MS) II, showed consistent enhancement of immunoglobulin G2a (IgG2a) production when it was in formulation with either ovalbumin or recombinant hemagglutinin B (rHagB) antigen. With rHagB antigen, it induced a significantly higher IgG2a response than the positive control GPI-0100, a well-studied semisynthetic saponin adjuvant mixture derived from Quillaja saponaria Molina saponins, known for its ability to induce a balanced Th1/Th2 immunity. These results confirm that Momordica saponins are a viable natural source to provide potent saponin adjuvants after simple chemical derivatization and identify VSA-2 (5b) as another MS-based promising immunostimulant lead owing to its distinctive ability in potentiating the IgG2a response.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.W. is inventor on patent applications based on this work.
Figures
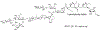



References
-
- Kensil CR; Mo AX; Truneh A Current vaccine adjuvants: An overview of a diverse class. Frontiers in Bioscience 2004, 9, 2972–2988. - PubMed
-
- Leroux-Roels G Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 2010, 28 Suppl 3, C25–36. - PubMed
-
- Sharp FA; Lavelle EC: Discovery of Vaccine Adjuvants. In Development of Therapeutic Agents Handbook; 1st ed.; Gad SC, Ed.; John Wiley & Sons, Inc: Hoboken, NJ, 2012; pp 533–546.
-
- Wang W Selection of adjuvants for enhanced vaccine potency. World J. Vaccines 2011, 01, 33–78.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

