Ca2+ mobilization-dependent reduction of the endoplasmic reticulum lumen is due to influx of cytosolic glutathione
- PMID: 32101139
- PMCID: PMC7043043
- DOI: 10.1186/s12915-020-0749-y
Ca2+ mobilization-dependent reduction of the endoplasmic reticulum lumen is due to influx of cytosolic glutathione
Abstract
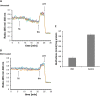
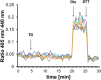
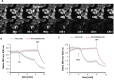
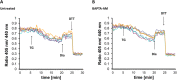
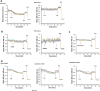
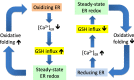
Background: The lumen of the endoplasmic reticulum (ER) acts as a cellular Ca2+ store and a site for oxidative protein folding, which is controlled by the reduced glutathione (GSH) and glutathione-disulfide (GSSG) redox pair. Although depletion of luminal Ca2+ from the ER provokes a rapid and reversible shift towards a more reducing poise in the ER, the underlying molecular basis remains unclear.
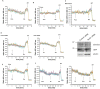
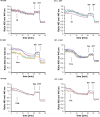
Results: We found that Ca2+ mobilization-dependent ER luminal reduction was sensitive to inhibition of GSH synthesis or dilution of cytosolic GSH by selective permeabilization of the plasma membrane. A glutathione-centered mechanism was further indicated by increased ER luminal glutathione levels in response to Ca2+ efflux. Inducible reduction of the ER lumen by GSH flux was independent of the Ca2+-binding chaperone calreticulin, which has previously been implicated in this process. However, opening the translocon channel by puromycin or addition of cyclosporine A mimicked the GSH-related effect of Ca2+ mobilization. While the action of puromycin was ascribable to Ca2+ leakage from the ER, the mechanism of cyclosporine A-induced GSH flux was independent of calcineurin and cyclophilins A and B and remained unclear.
Conclusions: Our data strongly suggest that ER influx of cytosolic GSH, rather than inhibition of local oxidoreductases, is responsible for the reductive shift upon Ca2+ mobilization. We postulate the existence of a Ca2+- and cyclosporine A-sensitive GSH transporter in the ER membrane. These findings have important implications for ER redox homeostasis under normal physiology and ER stress.
Keywords: Calcium; Calreticulin; Cyclophilins; Cyclosporine A; Endoplasmic reticulum; Endoplasmic reticulum stress; Glutathione; Membrane transport proteins; Redox homeostasis; Sec61 translocon.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Regulation of calcium homeostasis and flux between the endoplasmic reticulum and the cytosol.J Biol Chem. 2022 Jul;298(7):102061. doi: 10.1016/j.jbc.2022.102061. Epub 2022 May 21. J Biol Chem. 2022. PMID: 35609712 Free PMC article. Review.
-
Retarded PDI diffusion and a reductive shift in poise of the calcium depleted endoplasmic reticulum.BMC Biol. 2015 Jan 10;13:2. doi: 10.1186/s12915-014-0112-2. BMC Biol. 2015. PMID: 25575667 Free PMC article.
-
Endoplasmic Reticulum Transport of Glutathione by Sec61 Is Regulated by Ero1 and Bip.Mol Cell. 2017 Sep 21;67(6):962-973.e5. doi: 10.1016/j.molcel.2017.08.012. Epub 2017 Sep 14. Mol Cell. 2017. PMID: 28918898 Free PMC article.
-
Oxidative protein folding and unfolded protein response elicit differing redox regulation in endoplasmic reticulum and cytosol of yeast.Free Radic Biol Med. 2012 May 1;52(9):2000-12. doi: 10.1016/j.freeradbiomed.2012.02.048. Epub 2012 Mar 8. Free Radic Biol Med. 2012. PMID: 22406321
-
ER stress as a sentinel mechanism for ER Ca2+ homeostasis.Cell Calcium. 2024 Dec;124:102961. doi: 10.1016/j.ceca.2024.102961. Epub 2024 Oct 18. Cell Calcium. 2024. PMID: 39471738 Review.
Cited by
-
Necrosis Links Neurodegeneration and Neuroinflammation in Neurodegenerative Disease.Int J Mol Sci. 2024 Mar 24;25(7):3636. doi: 10.3390/ijms25073636. Int J Mol Sci. 2024. PMID: 38612448 Free PMC article. Review.
-
The mammalian peroxisomal membrane is permeable to both GSH and GSSG - Implications for intraperoxisomal redox homeostasis.Redox Biol. 2023 Jul;63:102764. doi: 10.1016/j.redox.2023.102764. Epub 2023 May 25. Redox Biol. 2023. PMID: 37257275 Free PMC article.
-
A Naturally Derived Watercress Flower-Based Phenethyl Isothiocyanate-Enriched Extract Induces the Activation of Intrinsic Apoptosis via Subcellular Ultrastructural and Ca2+ Efflux Alterations in an In Vitro Model of Human Malignant Melanoma.Nutrients. 2023 Sep 18;15(18):4044. doi: 10.3390/nu15184044. Nutrients. 2023. PMID: 37764828 Free PMC article.
-
Glutathione dynamics in subcellular compartments and implications for drug development.Curr Opin Chem Biol. 2024 Aug;81:102505. doi: 10.1016/j.cbpa.2024.102505. Epub 2024 Jul 24. Curr Opin Chem Biol. 2024. PMID: 39053236 Free PMC article. Review.
-
Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum.Cells. 2021 Jan 25;10(2):233. doi: 10.3390/cells10020233. Cells. 2021. PMID: 33504070 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

