Optimal Antithrombotic Regimens for Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: An Updated Network Meta-analysis
- PMID: 32101251
- PMCID: PMC7240352
- DOI: 10.1001/jamacardio.2019.6175
Optimal Antithrombotic Regimens for Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: An Updated Network Meta-analysis
Abstract
Importance: Antithrombotic treatment in patients with atrial fibrillation (AF) and percutaneous coronary intervention (PCI) presents a balancing act with regard to bleeding and ischemic risks.
Objectives: To evaluate the safety and efficacy of 4 antithrombotic regimens by conducting an up-to-date network meta-analysis and to identify the optimal treatment for patients with AF undergoing PCI.
Data sources: Online computerized database (MEDLINE).
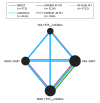
Study selection: Five randomized studies were included (N = 11 542; WOEST, PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS, ENTRUST-AF PCI).
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in this network meta-analysis, in which bayesian random-effects models were applied. The data were analyzed from September 9 to 29, 2019.
Main outcomes and measures: The primary safety outcome was thrombolysis in myocardial infarction (TIMI) major bleeding and the primary efficacy outcome was trial-defined major adverse cardiovascular events (MACE).
Results: The total number of participants included in the study was 11 532. The mean age of the participants ranged from 70 to 72 years, 69% to 83% were male, 20% to 26% were female, and the participants were predominantly white (>90%). Compared with vitamin K antagonists (VKA) plus dual antiplatelet therapy (DAPT) (reference), the odds ratios (ORs) (95% credible intervals) for TIMI major bleeding were 0.57 (0.31-1.00) for VKA plus P2Y12 inhibitor, 0.69 (0.40-1.16) for non-VKA oral anticoagulant (NOAC) plus DAPT, and 0.52 (0.35-0.79) for NOAC plus P2Y12 inhibitor. For MACE, using VKA plus DAPT as reference, the ORs (95% credible intervals) were 0.97 (0.64-1.42) for VKA plus P2Y12 inhibitor, 0.95 (0.64-1.39) for NOAC plus DAPT, and 1.03 (0.77-1.38) for NOAC plus P2Y12 inhibitor.
Conclusions and relevance: The findings of this study suggest that an antithrombotic regimen of VKA plus DAPT should generally be avoided, because regimens in which aspirin is discontinued may lead to lower bleeding risk and no difference in antithrombotic effectiveness. The use of a NOAC plus a P2Y12 inhibitor without aspirin may be the most favorable treatment option and the preferred antithrombotic regimen for most patients with AF undergoing PCI.
Conflict of interest statement
Figures

Comment in
-
Antithrombotic Strategies in Patients With Atrial Fibrillation and Percutaneous Coronary Intervention.JAMA Cardiol. 2021 Feb 1;6(2):240-241. doi: 10.1001/jamacardio.2020.4753. JAMA Cardiol. 2021. PMID: 33026414 No abstract available.
-
Antithrombotic Strategies in Patients With Atrial Fibrillation and Percutaneous Coronary Intervention-Reply.JAMA Cardiol. 2021 Feb 1;6(2):241. doi: 10.1001/jamacardio.2020.4759. JAMA Cardiol. 2021. PMID: 33026416 No abstract available.
References
-
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with Atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 - DOI - PubMed
-
- Dewilde WJM, Oirbans T, Verheugt FWA, et al. ; WOEST Study Investigators . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115. doi: 10.1016/S0140-6736(12)62177-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

