Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial
- PMID: 32101256
- PMCID: PMC7142380
- DOI: 10.1001/jamadermatol.2020.0079
Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial
Abstract
Importance: Interleukin 13 (IL-13) is a central pathogenic mediator driving multiple features of atopic dermatitis (AD) pathophysiology.
Objective: To evaluate the efficacy and safety of lebrikizumab, a novel, high-affinity, monoclonal antibody targeting IL-13 that selectively prevents formation of the IL-13Rα1/IL-4Rα heterodimer receptor signaling complex, in adults with moderate to severe AD.
Design, setting, and participants: A phase 2b, double-blind, placebo-controlled, dose-ranging randomized clinical trial of lebrikizumab injections every 4 weeks or every 2 weeks was conducted from January 23, 2018, to May 23, 2019, at 57 US centers. Participants were adults 18 years or older with moderate to severe AD.
Interventions: Patients were randomized 2:3:3:3 to placebo every 2 weeks or to subcutaneous injections of lebrikizumab at the following doses: 125 mg every 4 weeks (250-mg loading dose [LD]), 250 mg every 4 weeks (500-mg LD), or 250 mg every 2 weeks (500-mg LD at baseline and week 2).
Main outcomes and measures: The primary end point was percentage change in the Eczema Area and Severity Index (EASI) (baseline to week 16). Secondary end points for week 16 included proportion of patients achieving Investigator's Global Assessment score of 0 or 1 (IGA 0/1); EASI improvement of at least 50%, 75%, or 90% from baseline; percentage change in the pruritus numeric rating scale (NRS) score; and pruritus NRS score improvement of at least 4 points. Safety assessments included treatment-emergent adverse events.
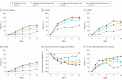
Results: A total of 280 patients (mean [SD] age, 39.3 [17.5] years; 166 [59.3%] female) were randomized to placebo (n = 52) or to lebrikizumab at doses of 125 mg every 4 weeks (n = 73), 250 mg every 4 weeks (n = 80), or 250 mg every 2 weeks (n = 75). Compared with placebo (EASI least squares mean [SD] percentage change, -41.1% [56.5%]), lebrikizumab groups showed dose-dependent, statistically significant improvement in the primary end point vs placebo at week 16: 125 mg every 4 weeks (-62.3% [37.3%], P = .02), 250 mg every 4 weeks (-69.2% [38.3%], P = .002), and 250 mg every 2 weeks (-72.1% [37.2%], P < .001). Differences vs placebo-treated patients (2 of 44 [4.5%]) in pruritus NRS improvement of at least 4 points were seen as early as day 2 in the high-dose lebrikizumab group (9 of 59 [15.3%]). Treatment-emergent adverse events were reported in 24 of 52 placebo patients (46.2%) and in lebrikizumab patients as follows: 42 of 73 (57.5%) for 125 mg every 4 weeks, 39 of 80 (48.8%) for 250 mg every 4 weeks, and 46 of 75 (61.3%) for 250 mg every 2 weeks; most were mild to moderate and did not lead to discontinuation. Low rates of injection-site reactions (1 of 52 [1.9%] in the placebo group vs 13 of 228 [5.7%] in all lebrikizumab groups), herpesvirus infections (2 [3.8%] vs 8 [3.5%]), and conjunctivitis (0% vs 6 [2.6%]) were reported.
Conclusions and relevance: During 16 weeks of treatment, lebrikizumab provided rapid, dose-dependent efficacy across a broad range of clinical manifestations in adult patients with moderate to severe AD and demonstrated a favorable safety profile. These data support the central role of IL-13 in AD pathophysiology. If these findings replicate in phase 3 studies, lebrikizumab may meaningfully advance the standard of care for moderate to severe AD.
Trial registration: ClinicalTrials.gov Identifier: NCT03443024.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

