Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity
- PMID: 32101357
- PMCID: PMC7106961
- DOI: 10.1111/cpr.12783
Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity
Abstract
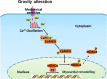
Objectives: Cardiac Ca2+ signalling plays an essential role in regulating excitation-contraction coupling and cardiac remodelling. However, the response of cardiomyocytes to simulated microgravity and hypergravity and the effects on Ca2+ signalling remain unknown. Here, we elucidate the mechanisms underlying the proliferation and remodelling of HL-1 cardiomyocytes subjected to rotation-simulated microgravity and 4G hypergravity.
Materials and methods: The cardiomyocyte cell line HL-1 was used in this study. A clinostat and centrifuge were used to study the effects of microgravity and hypergravity, respectively, on cells. Calcium signalling was detected with laser scanning confocal microscopy. Protein and mRNA levels were detected by Western blotting and real-time PCR, respectively. Wheat germ agglutinin (WGA) staining was used to analyse cell size.
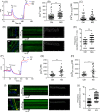
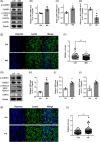
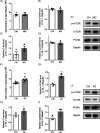
Results: Our data showed that spontaneous calcium oscillations and cytosolic calcium concentration are both increased in HL-1 cells after simulated microgravity and 4G hypergravity. Increased cytosolic calcium leads to activation of calmodulin-dependent protein kinase II/histone deacetylase 4 (CaMKII/HDAC4) signalling and upregulation of the foetal genes ANP and BNP, indicating cardiac remodelling. WGA staining indicated that cell size was decreased following rotation-simulated microgravity and increased following 4G hypergravity. Moreover, HL-1 cell proliferation was increased significantly under hypergravity but not rotation-simulated microgravity.
Conclusions: Our study demonstrates for the first time that Ca2+ /CaMKII/HDAC4 signalling plays a pivotal role in myocardial remodelling under rotation-simulated microgravity and hypergravity.
Keywords: Ca2+; cardiac remodelling; hypergravity; microgravity; proliferation.
© 2020 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures

Similar articles
-
Sperm Motility of Mice under Simulated Microgravity and Hypergravity.Int J Mol Sci. 2020 Jul 17;21(14):5054. doi: 10.3390/ijms21145054. Int J Mol Sci. 2020. PMID: 32709012 Free PMC article.
-
Dome formation and tubule morphogenesis by Xenopus kidney A6 cell cultures exposed to microgravity simulated with a 3D-clinostat and to hypergravity.In Vitro Cell Dev Biol Anim. 2001 Jan;37(1):31-44. doi: 10.1290/1071-2690(2001)037<0031:dfatmb>2.0.co;2. In Vitro Cell Dev Biol Anim. 2001. PMID: 11249203
-
Simulated Microgravity and Hypergravity Affect the Expression Level of Soluble Guanylate Cyclase, Adenylate Cyclase, and Phosphodiesterase Genesin Rat Ventricular Cardiomyocytes.Bull Exp Biol Med. 2024 Jan;176(3):359-362. doi: 10.1007/s10517-024-06024-z. Epub 2024 Feb 12. Bull Exp Biol Med. 2024. PMID: 38342810
-
The impact of microgravity and hypergravity on endothelial cells.Biomed Res Int. 2015;2015:434803. doi: 10.1155/2015/434803. Epub 2015 Jan 13. Biomed Res Int. 2015. PMID: 25654101 Free PMC article. Review.
-
CaMKIIδ and cardiomyocyte Ca(2+) signalling new perspectives on splice variant targeting.Clin Exp Pharmacol Physiol. 2015 Dec;42(12):1327-32. doi: 10.1111/1440-1681.12489. Clin Exp Pharmacol Physiol. 2015. PMID: 26361740 Review.
Cited by
-
Ckip-1 3'-UTR Attenuates Simulated Microgravity-Induced Cardiac Atrophy.Front Cell Dev Biol. 2022 Feb 2;9:796902. doi: 10.3389/fcell.2021.796902. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35186951 Free PMC article.
-
Cardiac function, structural, and electrical remodeling by microgravity exposure.Am J Physiol Heart Circ Physiol. 2023 Jan 1;324(1):H1-H13. doi: 10.1152/ajpheart.00611.2022. Epub 2022 Nov 18. Am J Physiol Heart Circ Physiol. 2023. PMID: 36399385 Free PMC article. Review.
-
Blueprints for Constructing Microgravity Analogs.Methods Mol Biol. 2022;2368:215-232. doi: 10.1007/978-1-0716-1677-2_14. Methods Mol Biol. 2022. PMID: 34647258
-
Effects of Proton Therapy on Cardiac Fibrosis, Calcium Homeostasis, and AQP4 Expression in Hypergravity-Exposed Rats.Int J Mol Sci. 2025 Jun 30;26(13):6326. doi: 10.3390/ijms26136326. Int J Mol Sci. 2025. PMID: 40650101 Free PMC article.
-
Histone deacetylase 4 and 5 translocation elicited by microsecond pulsed electric field exposure is mediated by kinase activity.Front Bioeng Biotechnol. 2022 Nov 17;10:1047851. doi: 10.3389/fbioe.2022.1047851. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36466344 Free PMC article.
References
-
- Hughson RL, Helm A, Durante M. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol. 2018;15:167‐180. - PubMed
-
- Costa‐Almeida R, Granja PL, Gomes ME. Gravity, tissue engineering, and the missing link. Trends Biotechnol. 2018;36:343‐347. - PubMed
-
- Zayzafoon M, Meyers VE, McDonald JM. Microgravity: the immune response and bone. Immunol Rev. 2005;208:267‐280. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

